Uncertainty exists about the long-term safety and efficacy outcomes of sirolimus-eluting stents (SESs) in unselected patients. The present study was performed to evaluate the safety and efficacy of the SES in treatment of patients with coronary artery disease in an unselected population. Over a 2-year period, 1,504 consecutive patients undergoing percutaneous coronary intervention with ≥1 SES were enrolled. The primary end point was the occurrence of target vessel failure (TVF; a composite of cardiac death, myocardial infarction, or clinically driven target vessel revascularization). An independent clinical event committee adjudicated all adverse events up to 2-year follow-up. Dual antiplatelet therapy was recommended for ≥1 year throughout the study period. Mean age was 65 ± 11 years; 75% were men, and 34% were diabetics. SESs were implanted for off-label indications in 86% of cases. TVF rates were 3.3%, 6.9%, 11.5%, and 15.5% at 30-day, 6-month, 1-year, and 2-year follow-ups, respectively. The 2-year cumulative rate of definite/probable stent thrombosis was 0.9%; 0.2% was very late thrombosis, occurring from 1 year to 2 years. Patients off dual antiplatelet therapy at 6 months had a significantly increased rate of subsequent death from noncardiac causes. Patients off dual antiplatelet therapy at 1 year had a significantly decreased rate of subsequent clinically driven target lesion revascularization. In conclusion, use of SESs in unselected patients with coronary artery disease was associated with a low TVF rate at 2 years with an acceptable incidence of stent thrombosis.
The United States Food and Drug Administration (FDA) approved the use of drug-eluting stents (DESs) for treatment of coronary artery disease based on results of randomized controlled trials showing a significant decrease in restenosis and need for repeat revascularization with DESs compared to bare metal stents. Typically, randomized pivotal trials have excluded patients with complex coronary artery disease with high risk for cardiac events. In clinical practice, DESs have also been used for off-label indications. However, expanded use of DES in everyday clinical practice is less well studied, and the possibility of unrecognized complications may exist. The Comprehensive Assessment of Sirolimus-Eluting Stents in Complex Lesions (MATRIX) registry was designed to evaluate the safety and efficacy of the sirolimus-eluting stent (SES) in an unselected population of patients with obstructive coronary artery disease. The SES was the only DES approved by the FDA at the time of study initiation. This report focuses on clinical outcomes in the MATRIX registry up to 2-year follow-up.
Methods
The MATRIX registry was conducted under an FDA-approved investigative device exemption and was a prospective, open-label, nonrandomized registry of 1,504 consecutive patients ≥18 years undergoing percutaneous coronary intervention (PCI) requiring the placement of ≥1 DES (Cypher, Cordis, Johnson and Johnson, Warren, New Jersey) for single- or multivessel coronary artery disease. Inclusion criteria were (1) ≥1 lesion with ≥50% diameter stenosis in a native coronary artery or a bypass graft requiring PCI with stenting not to exceed 108 mm of stent length, (2) de novo and restenotic lesions including in stent restenosis and radiation failure, (3) reference diameter from 2.5 to 3.5 mm, and (4) ability to understand and grant written informed consent. Exclusion criteria were (1) confirmed pregnancy at time of index PCI, (2) known allergies to aspirin, clopidogrel, or ticlopidine, (3) known allergies to heparin and bivalirudin, (4) known allergy to any component of a SES, and (5) a significant medical condition that in the investigators’ opinion might interfere with a patient’s optimal participation in this study. From March 2004 to August 2006, consecutive patients (n = 1,504) who underwent PCI with the approved SES at the 2 participating sites (Lenox Hill Hospital, New York, New York, and Columbia University Medical Center, New York, New York) were considered for enrollment in this study. The respective institutional review boards approved the protocol and all patients granted written informed consent.
PCI and stent implantation were performed in the standard manner. Heparin was administered to maintain an activated clotting time >250 seconds, and bivalirudin was used as an alternative anticoagulant in most cases (85%) according to standard clinical practice in the 2 clinical sites. After intracoronary injection of nitroglycerin, pre- and postprocedural angiographies of the involved vessel(s) were performed in ≥2 near orthogonal views showing the target lesion free of foreshortening or vessel overlap to allow for accurate quantitative coronary angiographic measurements. Pre- and postdilatation were performed at the operator’s discretion. In the event of an additional stent requirement, an SES was used. The following SES sizes were used in the MATRIX registry: 8, 18, 23, and 33 mm in length, with diameters of 2.5, 3.0, and 3.5 mm. Use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the operator. Successful stent implantation was defined as the achievement of a final diameter stenosis <50% by quantitative coronary angiography after stent implantation with normal flow. According to protocol, physicians prescribed aspirin 325 mg/day for 1 month, 81 mg/day thereafter plus clopidogrel 75 mg/day for ≥1 year after the procedure. Ticlopidine was an option for possible clopidogrel allergy. A patient was provided with the prescription and a written instruction sheet. General practitioners and general cardiologists were informed by written transmission of the preliminary and final procedure reports that included information on dose and duration of dual antiplatelet therapy.
Follow-up was planned at 30 days, 6 months, 1 year, and 2 years after the index procedure; at these respective time points follow-up was available for 99.1%, 97.8%, 95.5%, and 85.3% of patients. At each follow-up time point, information was collected on antiplatelet medication adherence and occurrence of clinical events. All clinical end points (see below) were adjudicated by an independent clinical events committee.
The primary end point was the occurrence of target vessel failure (TVF), a composite of cardiac death, Q-wave- and non–Q-wave myocardial infarctions, or clinically driven target vessel revascularization. Q-wave myocardial infarction was defined as the development of new pathologic Q waves ≥0.04 second in duration in ≥2 contiguous leads as assessed by the electrocardiographic core laboratory with creatine kinase or creatine kinase-MB levels increased above normal. Non–Q-wave myocardial infarction was defined as an increase of creatine kinase levels to >2 times the upper normal limit with increased creatine kinase-MB in the absence of new pathologic Q waves. Target vessel revascularization was considered clinically driven in patients with a positive functional study result, ischemic changes on electrocardiogram consistent with the target vessel, an in-lesion diameter stenosis ≥50% by quantitative coronary angiography if the patient has ischemic symptoms, or an in-lesion diameter stenosis ≥70% by quantitative coronary angiography in the absence of ischemic symptoms. Secondary clinical endpoints included rates of individual clinical events and stent thrombosis. Stent thrombosis was categorized according to definitions proposed by the Academic Research Consortium as definite or probable stent thrombosis. Timing of stent thrombosis was classified as acute (<24 hours), subacute (24 hours to 30 days), late (1 month to 1 year), and very late (>1 year).
Continuous variables were summarized using mean ± SD. Categorical variables were summarized using frequencies. Survival curves using all available follow-up data were constructed for time-to-event variables using the Kaplan-Meier method and compared by log-rank test. Data on patients who were lost to follow-up were censored at the time of the last contact. To investigate the impact of cessation of dual antiplatelet therapy on subsequent clinical events, we performed landmark analyses comparing event rates (death, myocardial infarction, and clinically driven target vessel revascularization) between patients on and off dual antiplatelet therapy. Two landmark time points were considered, 6 months and 1 year. Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
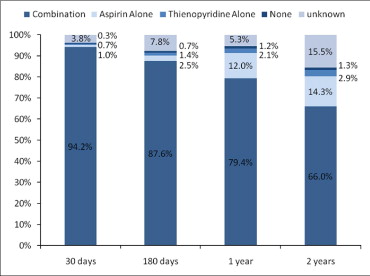
In total 1,504 patients were enrolled in the MATRIX registry; mean age was 65 ± 11 years, 75% were men, and 34% had diabetes mellitus. Additional baseline characteristics are listed in Table 1 . SESs were successfully implanted in 98.6% of lesions and a mean of 2.1 ± 1.2 SESs (per patient) was implanted during the index procedure. Table 2 lists procedural characteristics for the study cohort. Most patients (86%) underwent stenting for off-label indications, including multivessel stenting (n = 462, 30.7%), bifurcation lesions (n = 295, 19.6%), saphenous vein grafts (n = 67, 4.5%), long-term total occlusions (n = 58, 3.9%), and acute myocardial infarction (n = 49, 3.3%). At time of hospital discharge, 99.6% of patients were being treated with aspirin and 99.6% of patients were treated with clopidogrel or ticlopidine. Figure 1 shows the proportion of patients on aspirin and clopidogrel or ticlopidine during follow-up; high levels of adherence were documented.
| Age (years) | 64.8 ± 11.4 |
| Body mass index (kg/m 2 ) | 29 ± 5.6 |
| Man | 74.5% |
| Diabetes mellitus | 33.7% |
| Insulin-dependent diabetes mellitus | 7.2% |
| Hypertension ⁎ | 82.5% |
| Hyperlipidemia † | 84.7% |
| Smoker | 10.9% |
| Previous myocardial infarction | 33.2% |
| Previous percutaneous coronary intervention | 44.1% |
| Previous coronary artery bypass grafting | 20.9% |
| Indication for index procedure | |
| Stable angina with abnormal stress test | 63.7% |
| Unstable angina pectoris | 33.2% |
| Acute myocardial infarction | 3.4% |
| Number of coronary arteries narrowed | 2.0 ± 0.9 |
| 1 | 35.8% |
| 2 | 33.2% |
| ≥3 | 31.0% |
| Moderate/severe left ventricular dysfunction | 8.2% |
⁎ Defined as a documented history of hypertension diagnosed and/or treated by a physician.
† Defines as a documented history of hyperlipidemia diagnosed and/or treated by a physician.
| Number of lesions treated | 2,879 |
| Number of lesions treated per patient | 1.9 ± 1.0 |
| Number of vessels treated per patient | 1.3 ± 0.5 |
| Treated vessel | |
| Right coronary artery | 32.0% |
| Left anterior descending coronary artery | 44.5% |
| Left circumflex coronary artery | 35.4% |
| Ramus intermedius | 4.4% |
| Left main coronary artery | 3.3% |
| Saphenous vein graft | 4.5% |
| Arterial bypass graft | 0.6% |
| Lesion length (mm) | 18.0 ± 9.8 |
| Reference vessel diameter (mm) | 3.00 ± 0.46 |
| Preprocedural minimum lumen diameter (mm) | 0.68 ± 0.39 |
| Postprocedural minimum lumen diameter (mm) | 2.34 ± 0.43 |
| Acute gain (mm) | 1.67 ± 0.48 |
| American Heart Association/American College of Cardiology lesion type B2/C | 66.6% |
| Number of stents per patient | 2.1 ± 1.2 |
| Number of stents per lesion | 1.1 ± 0.5 |
| Stent length per lesion (mm) | 24.1 ± 12.7 |
| Stent diameter (mm) | 3.03 ± 0.42 |
| Predilatation performed | 64.7% |
| Postdilatatation performed | 37.6% |
| Maximum inflation pressure (atm) | 15.5 ± 2.6 |
Rates of cardiac death, myocardial infarction, and ischemia-driven target vessel revascularization and the composite of TVF at 30-day, 6-month, 1-year, and 2-year follow-up are shown in Figure 2 .
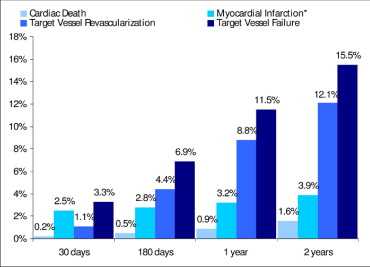
Figure 3 shows TVF rates at 2 years for selected high-risk subgroups (multivessel stenting, diabetes mellitus, bifurcation lesions, saphenous vein grafts, long-term total occlusions, acute myocardial infarction, and previous brachytherapy). The highest 2-year TVF rate (39.0%) was observed in the brachytherapy group due to high rates of clinically driven target vessel revascularization (35.8%) and stent thrombosis (6.9%). In the diabetic subgroup we observed a trend toward a higher incidence of TVF at 2 years in insulin-treated diabetics compared to noninsulin-treated diabetics (24.9% vs 18.7%, p = 0.11).
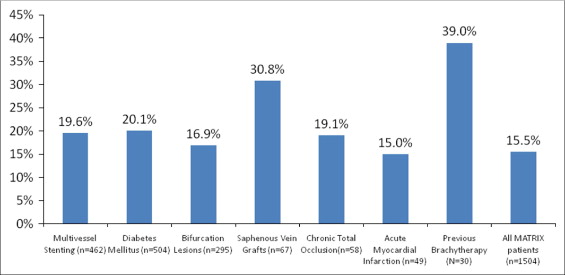
Figure 4 shows the timing of definite/probable stent thrombosis events and whether a patient was using dual antiplatelet therapy at time of stent thrombosis. Rates of acute, subacute, late, and very late definite/probable stent thrombosis were 0%, 0.3%, 0.3%, and 0.2%, respectively. Of the 13 patents with stent thrombosis, 10 patients (77%) had the event while on confirmed dual antiplatelet therapy and 3 patients (23%) while at an unknown antiplatelet therapy status.
Antiplatelet therapy follow-up information was available for 88.8% and 89.5% of patients at 6 months and 1 year, respectively. Table 3 presents results of landmark analyses comparing event rates between patients on and off dual antiplatelet therapy. Patients off dual antiplatelet therapy at 6 months had a significantly increased rate of subsequent death from noncardiac causes. Patients off dual antiplatelet therapy at 1 year had a significantly decreased rate of subsequent clinically driven target lesion revascularization.
| On | Off | p Value | |
|---|---|---|---|
| Landmark set at 6 months | |||
| Subjects | 1,199 | 45 | |
| Death | 2.5% | 8.0% | 0.047 |
| From cardiac causes | 1.0% | 0.0% | 0.539 |
| From noncardiac causes | 1.6% | 8.0% | <0.001 |
| Myocardial infarction | 1.1% | 0.0% | 0.519 |
| Target vessel revascularization | 8.7% | 0.0% | 0.056 |
| Target vessel failure | 9.9% | 0.0% | 0.041 |
| Landmark set at 1 yr | |||
| Subjects | 1,106 | 180 | |
| Death | 1.5% | 2.9% | 0.166 |
| From cardiac causes | 0.6% | 1.2% | 0.383 |
| From noncardiac causes | 0.9% | 1.8% | 0.152 |
| Myocardial infarction | 0.7% | 1.2% | 0.490 |
| Target vessel revascularization | 4.7% | 0.6% | 0.0279 |
| Target vessel failure | 5.5% | 3.0% | 0.179 |
Results
In total 1,504 patients were enrolled in the MATRIX registry; mean age was 65 ± 11 years, 75% were men, and 34% had diabetes mellitus. Additional baseline characteristics are listed in Table 1 . SESs were successfully implanted in 98.6% of lesions and a mean of 2.1 ± 1.2 SESs (per patient) was implanted during the index procedure. Table 2 lists procedural characteristics for the study cohort. Most patients (86%) underwent stenting for off-label indications, including multivessel stenting (n = 462, 30.7%), bifurcation lesions (n = 295, 19.6%), saphenous vein grafts (n = 67, 4.5%), long-term total occlusions (n = 58, 3.9%), and acute myocardial infarction (n = 49, 3.3%). At time of hospital discharge, 99.6% of patients were being treated with aspirin and 99.6% of patients were treated with clopidogrel or ticlopidine. Figure 1 shows the proportion of patients on aspirin and clopidogrel or ticlopidine during follow-up; high levels of adherence were documented.



