Chapter 31 Tuberculosis and Nontuberculous Mycobacterial Infections
Tuberculosis
Epidemiology, Risk Factors, and Pathogenesis
Epidemiology
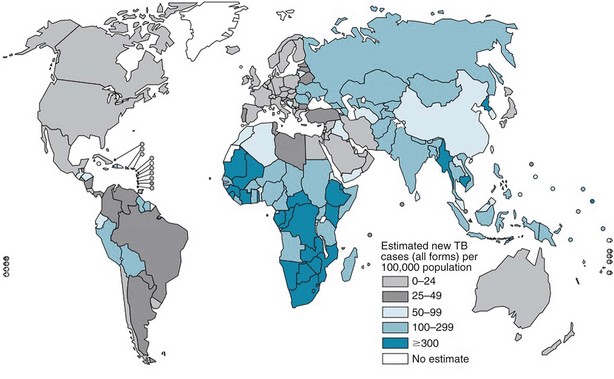
The burden of TB varies significantly throughout the world, with more than 90% of cases occurring among people residing in developing countries (Figure 31-1). The highest incidence rates for TB are in sub-Saharan Africa, particularly in the southern region of the continent. Not surprisingly, the highest prevalence of HIV co-infection also is in this region. Worldwide, approximately 23% of all persons with TB have underlying HIV co-infection; however, in sub-Saharan Africa, an estimated 50% of persons with TB have HIV/AIDS.
Risk Factors
Certain people are at higher risk for development of TB simply because they are more likely to be exposed to and thus infected with M. tuberculosis (Table 31-1). For example, approximately 60% of reported TB cases in the United States occur among foreign-born people who come from areas where the disease is endemic. Other populations with an increased prevalence of TB infection include certain racial and ethnic groups, low-income populations, the homeless, and injection drug users.
Table 31-1 Criteria for a Positive Reaction on Tuberculin Skin Testing
* The predominant chest radiographic finding consistent with previous tuberculosis is presence of fibrotic lesions; other changes such as pleural thickening or isolated calcified granulomas are not related.
† Medical conditions and factors associated with increased risk for development of active disease in a patient with latent tuberculosis infection include silicosis, end-stage renal disease, malnutrition, diabetes mellitus, carcinoma of the head or neck and lung, immunosuppressive therapy, lymphoma, leukemia, weight loss of more than 10% ideal body weight, gastrectomy, and jejunoileal bypass.
Anyone infected with M. tuberculosis can develop TB disease, but certain groups are at higher-than-normal risk for progression to active disease (see Table 31-1). Patients who have been recently infected with M. tuberculosis and those with medical conditions associated with significant immunosuppression are at particularly high risk for development of TB. HIV co-infection is the strongest known risk factor for the development of TB and is estimated to increase the risk of progression to TB by 50- to 100-fold. Inhibitors of tumor necrosis factor-α (TNF-α) may increase the risk for development of TB by up to 10-fold, and patients taking TNF-α blockers frequently present with disseminated disease. This association appears to be stronger for infliximab and adalimumab than for etanercept. Other medical conditions are associated with a more modest increase in risk for development of disease.
Pathogenesis
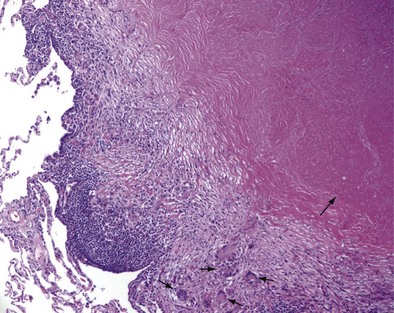
Once cell-mediated immunity develops, collections of activated T cells and macrophages form granulomas that wall off the mycobacterial organisms (Figure 31-2). For most persons with normal immune function, infection with M. tuberculosis seems to be arrested once cell-mediated immunity develops, even though small numbers of viable bacilli remain within the granuloma. Although a primary complex can sometimes be seen on chest radiograph, most TB infections are asymptomatic and can be detected only indirectly with a tuberculin skin test (TST) or interferon-γ release assay (IGRA). Persons with “walled-off” TB infection who do not have active disease are not infectious and thus cannot spread the disease to others.
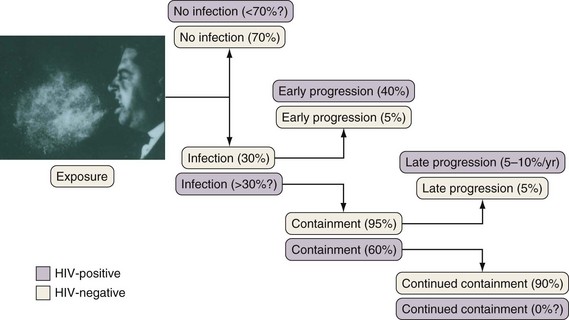
If cell-mediated immunity does not contain the tubercle bacilli, the initial infection progresses to active disease. Without treatment, infected persons have approximately a 5% chance of developing TB in the first 1 to 2 years after infection and an additional 5% chance of developing TB during the remainder of their lifetime (Figure 31-3). By contrast, persons who are co-infected with HIV have a 5% to 10% annual risk of active disease developing. When active TB develops soon after infection, the disease is referred to as primary TB. By contrast, when TB develops years or even decades after the initial infection, the disease is referred to as postprimary or reactivation disease. Exogenous reinfection, involving acquisition of a second strain of M. tuberculosis, also can lead to disease and seems to be more common in HIV-infected patients.
Clinical Features
Pulmonary Tuberculosis
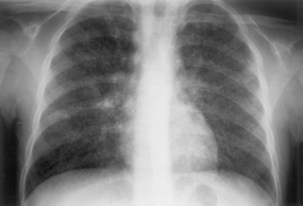
The initial infection in the lung, referred to as primary infection, causes formation of an inflammatory infiltrate, which may be seen on a chest radiograph, often in the middle or lower lung zones. The draining lymph nodes may enlarge and compress adjacent bronchi, particularly in infants and children. Parenchymal disease usually clears as cell-mediated immunity develops, and it tends to clear more rapidly than nodal involvement. If the parenchymal disease persists beyond the development of cell-mediated immunity, cavitation may occur, although this finding is uncommon. Pleural effusions are a common manifestation of primary TB and presumably result when a peripheral, caseous focus ruptures into the pleural space (Figure 31-4). Pleuritis caused by TB may manifest as an acute illness characterized by cough, fever, and pleuritic chest pain.
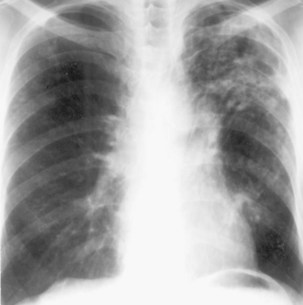
During most initial infections with M. tuberculosis, small numbers of organisms are disseminated hematogenously, and some become seeded in the apices of the lung. The organisms seem to grow preferentially in this well-oxygenated environment, with progression to active disease occurring months or years after the initial infection. This accounts for the characteristic radiographic location of reactivation disease, which in most cases occurs in the apical or posterior segments of the upper lobes (Figure 31-5). In areas of chronic infection or areas of caseation, fibrosis may occur. Fibrocaseous lesions may contain live mycobacteria for many years, and these are the lesions that may reactivate years later.
Miliary Tuberculosis
Miliary or disseminated TB occurs when tubercle bacilli spread throughout the body, through the bloodstream, resulting in small (approximately 1 to 2 mm) granulomatous lesions. Miliary TB is seen more commonly in infants, children less than 4 years old, and in immunocompromised people. Disease can result from early dissemination after infection or later after reactivation and dissemination. Disseminated TB usually develops insidiously with systemic symptoms such as fever, weakness, weight loss, fatigue, and anorexia. Cough and dyspnea also may be prominent symptoms. The mean duration of symptoms approaches 16 weeks, but some patients may go undiagnosed for more than 2 years. The chest radiograph typically shows the classic “miliary” pattern of diffuse small nodules (Figure 31-6). Sputum AFB smears are positive in 20% to 25% of cases, and sputum is culture-positive for M. tuberculosis in up to 65% of cases. Bronchoscopy should be considered in patients who are unable to produce sputum or who have produced negative sputum smears. Other potential sources include urine, which is culture-positive in up to 25% of patients, and liver and bone marrow, which are culture-positive in up to 25% to 40%.
Diagnosis
To diagnose TB, the disease must first be suspected. TB should be suspected in certain high-risk groups reviewed previously (see Table 31-1) and when the clinical and/or radiographic presentation is consistent with TB. The medical history should elicit whether or not the person suspected of having TB has been exposed to M. tuberculosis or has a previous history of TB infection or disease. Symptoms at presentation will vary depending on the sites(s) of involvement and extent of disease as described previously. Guidelines suggest that all persons with an unexplained cough lasting 2 to 3 weeks or more be evaluated for TB. Of note, up to 20% of patients with pulmonary disease are asymptomatic. Findings at physical examination are rather nonspecific and will vary, depending on the site of involvement. Among HIV-infected patients, TB should be considered when any respiratory infection or fever of unknown origin occurs, because the risk for TB in this group is substantially elevated, and signs and symptoms of TB often are atypical.
Radiographic Examinations
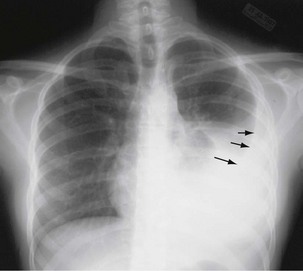
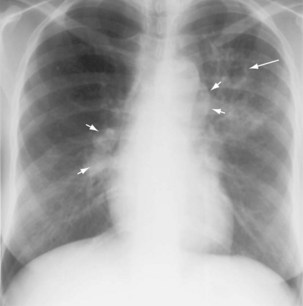
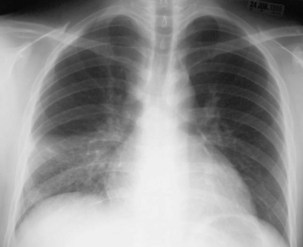
Plain chest radiography is a sensitive but nonspecific test to detect pulmonary TB. Radiographic manifestations of TB vary, depending on whether the patient has primary or postprimary TB and whether co-infection with HIV is present. Patients who have primary pulmonary TB at initial evaluation may demonstrate radiographic opacities in the lower lung zones and an associated pleural effusion (see Figure 31-4). TB caused by reactivation typically involves the apical and posterior segments of the upper lobes or superior segment of the lower lobe (see Figure 31-5). Cavitation and volume loss are common in reactivation disease but unusual in primary disease. Findings on the chest radiograph in patients co-infected with HIV depend on the severity of immunosuppression. Early in the course of HIV disease, the radiograph may show a typical reactivation pattern with cavitation (Figure 31-7), but as the CD4+ cell count declines, the radiographic appearance is more like the pattern seen in primary TB (Figure 31-8). Patients co-infected with HIV may sometimes have a normal-appearing chest radiograph despite being sputum AFB smear–positive.
Bacteriologic Examination
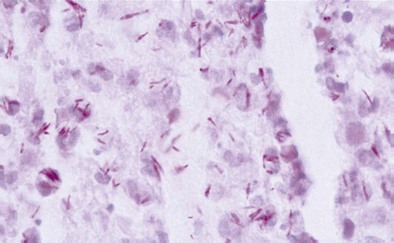
Sputum Microscopy
Diagnosis of pulmonary TB begins with obtaining two or three spontaneously expectorated sputum samples collected at 8- to 24-hour intervals, with at least one collected in early morning. Two methods are commonly used for acid-fast staining: the carbolfuchsin methods (Ziehl-Neelsen and Kinyoun methods) and a fluorochrome procedure that uses auramine O or auramine-rhodamine dyes (Figure 31-9).