12 Tricuspid Atresia
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
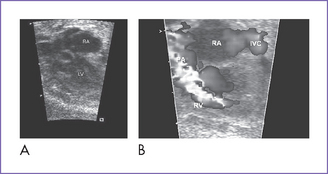
2. The four-chamber view is abnormal (Fig. 12-1), but cardiac axis, position, and size are normal (cardiothoracic ratio = 0.35).
3. The tricuspid valve is atretic (no valve tissue present).
4. The right ventricle (RV) is small (Fig. 12-2) and has mildly reduced function.
5. The left atrioventricular (AV) valve is patent and opens into a dominant left ventricle (LV) with a narrow jet of mitral regurgitation (4 m/s).
6. There is no evidence of endocardial fibroelastosis.
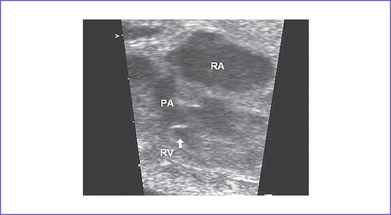
7. The outflow assessment reveals normally related great arteries with clear asymmetry in size (aorta–to–pulmonary artery diameter ratio = 1:0.5).
8. The pulmonary arteries are small and hypoplastic: The right pulmonary artery is 0.26 cm and the left is 0.25 cm. They are anterior and to the left of the aorta.
9. The ascending aorta is large (posterior and to the right) and has normal Doppler blood velocity with antegrade flow.
10. The pulmonary valve is bicuspid and domes mildly in systole.
11. The aortic annulus is of normal size and there is normal Doppler velocity through it.
12. The aortic arch is leftward and the ductal arch is slightly bigger than the aortic arch. The flow is reversed in the ductal arch (aorta to pulmonary) but is normal antegrade through the aortic arch.
13. There is a moderate-sized muscular ventricular septal defect (VSD) with left-to-right shunt to the pulmonary valve (see Fig. 12-1B).
14. The foramen ovale is unrestricted, with mainly right-to-left shunt and normal pulmonary venous flow pattern.
15. The LV Tei index (myocardial performance index) of 0.34 is normal.
D. Fetal management and counseling
1. Amniocentesis could be deferred in view of gestation and the low chance of having chromosomal anomalies with tricuspid atresia.
2. Follow-up included serial antenatal studies at 4-week intervals to assess:
b. Size and direction of flow across the foramen ovale. It could become restricted with an abnormal flow pattern, or the systemic venous Doppler could indicate increasing central venous pressure.
c. Progressive pulmonary outflow obstruction and diminution in VSD size.
d. Branch pulmonary artery growth.
e. Mitral valve anatomy and function.
f. Development of new lesions such as subpulmonary obstruction.
F. Neonatal management
a. Prostaglandin E1 (PGE1) infusion should be started to maintain ductal patency. The hyperoxia test could be deferred given the retrograde flow through the ductus arteriosus in utero consistent with critical pulmonary outflow obstruction.
b. Rarely, if the baby has relatively high systemic saturations but very poor perfusion and evolving evidence of right heart failure and poor cardiac output, an urgent balloon atrial septostomy is required to allow more mixing at the atrial level while surgery is arranged. At times, if the foramen ovale is mildly restrictive before birth, increased pulmonary venous return and higher left atrial pressures after birth can hamper the obligate right-to-left shunt and lead to increasing central venous pressures.
c. Infants with VSD of adequate size and adequate blood flow need to be followed closely for decreasing oxygen saturation resulting from reduction in the size of VSD.
G. Follow-up
1. Quality of life after the Fontan procedure is guarded.
2. Survival after the Fontan procedure (Fontan et al, 1991, results refer mainly to patients with tricuspid atresia).
3. Outcomes after the Fontan procedure have improved significantly over the past decades. Improvements in early survival are in part due to:
a. Better understanding of patient-related risk factors and their application to patient selection.
b. Patient preparation and optimized staging of the Fontan procedure.
c. Procedural modifications (e.g., lateral tunnel or extracardiac Fontan).
4. As patients grow up, they should be followed by an adult congenital heart disease cardiologist for life.
I. Outcome of this case
1. Labor was induced at 38 weeks’ gestation.
2. The baby was born with good Apgar scores and good weight.
3. Moderate cyanosis was noted at birth: Pulse oximeter reading was 77%.
4. Postnatal echocardiography confirmed the diagnosis of tricuspid atresia, hypoplastic right ventricle, and severe critical pulmonary stenosis with a large muscular VSD. There was a large atrial septal defect (ASD) and a patent ductus arteriosus with bidirectional shunting.
6. The baby had a palliative aortopulmonary shunt at I week of age.
7. At 7 months (6 kg), he had a successful bidirectional Glenn operation.
8. At the age of 2 years, after a diagnostic cardiac catheterization to measure pulmonary vascular resistance, he had a successful extracardiac Fontan operation.
9. Because this neonate was born with tricuspid atresia and pulmonary stenosis, the prognosis is the best of all the types of single ventricle. With a left ventricular pumping chamber, the long-term ventricular function is optimal.
II. YOUR HANDY REFERENCE
A. Tricuspid atresia
a. The incidence of tricuspid atresia has been reported at between 0.3% and 5.3% of infants with congenital heart disease.
b. In most reports, incidence is between 1% and 2.5%.
c. In Allan and Sharland’s (1992) fetal series, incidence is 4%. In this series, out of 84 fetuses with tricuspid atresia, 54 pregnancies were terminated, there were two intrauterine deaths and four infant deaths, and 22 babies survived.
a. The overall prognosis without treatment for children with tricuspid atresia is poor.
b. The surgical options are a single ventricle repair with a bidirectional Glenn at 3 to 6 months (surgical mortality is about 2%-3%), followed by a Fontan operation at 2 years (surgical mortality is 2%-5%).
c. Children with tricuspid atresia usually have the lowest risk in Fontan repair among children undergoing this type of repair.
3. Associated syndromes and extracardiac anomalies.
a. Extracardiac malformations are uncommon.
b. Chromosomal anomalies are uncommon.
a. Pulmonary atresia with intact interventricular septum, where the tricuspid valve is hypoplastic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree