Cardiac resynchronization therapy (CRT) increases cardiac performance in patients with heart failure, but its effect on arterial pressure is not well established. To determine the effect of CRT on systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) a systematic review using standard nomenclatures for CRT was done in Scopus (MEDLINE and Embase), Cochrane Controlled Trials Register, National Institutes of Health http://www.ClinicalTrials.gov database, and bibliography of select meta-analyses for studies evaluating CRT in patients with dilated cardiomyopathy. Two independent investigators extracted the articles based on predefined criteria. The primary outcome was difference in arterial pressure parameters from baseline to after CRT in nonrandomized cohort trials. This was then validated by comparing the change in arterial pressure between CRT and medical therapy groups in randomized controlled trials. A random-effects model was used for analyses. Analyses of 15 nonrandomized studies showed that CRT resulted in an increase (from baseline) in SBP by 4.4 mm Hg (95% confidence interval [CI] 0.8 to 8.0, p = 0.02), no change in DBP (p = 0.21), and an increase in PP by 2.8 mm Hg (95% CI 1.0 to 4.6, p = 0.003). Results from the 3 randomized controlled trials were concordant with an increase in SBP by 3.9 mm Hg (95% CI 1.1 to 6.8, p = 0.007), no effect on DBP (p = 0.40), and an increase in PP by 4.3 mm Hg (95% CI 4.1 to 4.5, p <0.001) compared to medical therapy. In conclusion, CRT is associated with a modest increase in SBP and PP in patients with heart failure.
More than 90% of patients with heart failure (HF) have a history of hypertension. In contrast, in severe HF decreasing left ventricular function is unable to sustain the high blood pressure (BP) despite compensatory mechanisms (salt and water retention, vasoconstriction, sympathetic stimulation and desensitization, cardiac hypertrophy, and cellular changes including appearance of slow myosin, prolongation of action potential, post-translational modifications in calcium handling proteins, and increase in collagen). Thus, cardiac output decreases in parallel with systolic BP (SBP) and pulse pressure (PP). In patients with HF, cardiac resynchronization therapy (CRT) improves left ventricular systolic function, HF symptoms, quality of life, exercise tolerance, maladaptive remodeling, morbidity (HF admissions), and mortality. American College of Cardiology/American Heart Association guidelines recommend (class I) CRT in patients with ejection fraction ≤35%, QRS duration ≥120 ms, sinus rhythm, and New York Heart Association class III/ambulatory class IV HF symptoms on optimal medical therapy. However, it is not known if this improvement in systolic function translates into an increase in BP. Limited data seem to suggest an increase in SBP but this has not been consistently reported or studied. We hypothesized that CRT with its associated improvement in cardiac function would result in an increase in BP profile. Thus, our primary objective was to evaluate the effect of CRT on SBP, diastolic BP (DBP), and PP.
Methods
Eligible studies were prospective nonrandomized cohort studies that reported BP profile at baseline and after CRT with first follow-up within 6 months of CRT or randomized controlled trials of CRT (with or without implantable cardioverter–defibrillator) that reported BP profile in the CRT and medical therapy groups. To avoid major medication changes over follow-up period and additional cardiovascular insults, a limit of 6 months of follow-up was used for nonrandomized cohort studies. Studies were identified by searching electronic databases, including Scopus (MEDLINE 1966 to October 2009, Embase 1980 to October 2009), Cochrane Trials Register, and National Institutes of Health http://www.ClinicalTrials.gov database (closed studies only) using the terms “cardiac resynchronization” or “biventricular pacing” or “biventricular pacemaker” or “multisite pacing” or “multisite pacemaker” or “dual-site pacing” or “dual-site pacemaker” or “left ventricular pacing” or “left ventricular pacemaker.” In addition, reference lists of select meta-analyses were searched for reports of relevant studies. Studies in which the intervention included revascularization (coronary artery bypass grafting or percutaneous coronary intervention) at the time of CRT were excluded as revascularization could have potentially confounded the effect of CRT on BP outcomes ( Figure 1 ).
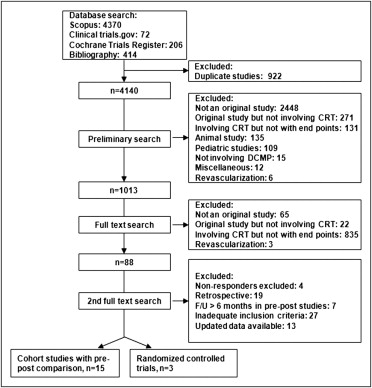
Eligible studies had to fulfill the following inclusion criteria: (1) randomized or nonrandomized cohort studies of CRT in patients with dilated cardiomyopathy, (2) studies reporting outcomes of interest (SBP, DBP, or PP; before and after CRT in nonrandomized cohort studies and CRT vs medical therapy in randomized controlled trials), and (3) follow-up <6 months for nonrandomized cohort studies. There were no restrictions based on language or year of publication. Studies were restricted to published data. Studies that had duplicated data, including same group of patients or for whom there were updated results available, were excluded. We included only studies that did not exclude nonresponders from their analyses to prevent bias towards a positive result. Further, studies including patients with ischemic and/or nonischemic cardiomyopathy were included in this meta-analysis, whereas studies evaluating the effect of CRT, specifically in pediatric patients, congenital heart disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy, chemotherapy-induced cardiomyopathy, and infectious cardiomyopathy, e.g., Chagas disease, were excluded.
Primary analyses were changes in BP parameters, i.e., SBP, DBP, and PP from baseline (before CRT) to that at follow-up (after CRT) in nonrandomized cohort studies. This was validated by comparing changes in BP parameters between a CRT group (with/without implantable cardioverter–defibrillator) and a medical therapy group (with/without implantable cardioverter–defibrillator) in randomized controlled trials. The 2 analyses were done separately without pooling the data.
Eligibility assessment and data abstraction were performed independently by 2 authors (S.V. and L.B.C) and supervised by S.A. We extracted inclusion criteria, exclusion criteria, baseline data, outcomes, and report quality. Disagreements were resolved by consensus.
To assess risk of bias in nonrandomized cohort studies, presence of single or double blinding and documentation of withdrawal were ascertained. For nonrandomized cohort studies, intermediate risk of bias was defined as a low possibility of bias in the 2 domains. As the studies were nonrandomized, none were considered at low risk.
For randomized controlled trials, methodologic quality was assessed by reported allocation generation, allocation concealment, blinding, documentation of withdrawal, selective reporting, and intention-to-treat analysis in line with the recommendation by the Cochrane Collaboration. For randomized controlled trials, high risk of bias was defined as a possibility of bias in ≥4 domains, moderate as a possibility of bias in 2 to 3 domains, and low risk as a possibility of bias in ≤1 domain.
Statistical analyses were done using Comprehensive Meta-Analysis 2.2.046 in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (for randomized controlled trials) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement for others. Mean difference was chosen as the principal measurement of effect as the unit of measurement was same across all studies. Studies included reported mean or difference in means and standard deviation (SD) or p value for the variables. If the SD was not available for 1 of the 3 variables (SBP, DBP, PP), then the SD was calculated using a before-versus-after correlation of 0.7 from the other 2 SDs available.
Data were analyzed for heterogeneity by I 2 statistic proposed by Higgins and Thompson separately for nonrandomized cohort studies and randomized controlled trials. Values <30% indicated mild heterogeneity and those >50% substantial heterogeneity. In the presence of heterogeneity, a random effects model (DerSimonian–Laird approach) was used to pool the data; otherwise, a fixed-effects model (inverse variance) was used. Publication bias was assessed and quantified using the regression intercept of Egger et al and corrected by the trim-and-fill method of Duval and Tweedie.
Results
Fifteen nonrandomized cohort studies and 3 randomized controlled trials met our inclusion criteria for analyses ( Figure 1 ). Of the 18 studies included in the meta-analyses, 15 (nonrandomized controlled trials) compared variables before and after CRT in 492 patients, whereas 3 studies (randomized controlled trials) compared CRT (n = 1,637) to optimal medical management (n = 727). Baseline characteristics and inclusion and exclusion criteria are presented in Tables 1 and 2 . There were 2 studies on the Pacing Therapies in Congestive Heart Failure (PATH-CHF) trial but separate variables were extracted from each study. Two trials with 3 published studies included patients with epicardial pacing, whereas the other studies used transvenous biventricular pacing.
| Study | Subjects | Age (years) | NYHA Class | Baseline EF (%) | QRS Width (ms) | Sinus Rhythm (%) | Ischemic Cause (%) | Men (%) | EF After CRT |
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials ⁎ | |||||||||
| Piccirillo et al, 2006 | 15/16 | 65/65 | 3.7/3.7 | 0.22/0.23 | 159/160 | 1/1 | 1/1 | 0.80/0.81 | 0.28/0.22 |
| Bristow et al, 2004 | 308/1,212 | 68/67 | 3.2/3.1 | 0.22/0.21 | 158/160 | 1/1 | 0.59/0.54 | 0.69/0.67 | NA |
| Cleland et al, 2005 | 404/409 | 66/67 | 3.1/3.1 | 0.25/0.25 | 160/160 | 1/1 | 0.36/0.40 | 0.73/0.74 | NA |
| Nonrandomized cohort studies | |||||||||
| Auricchio et al, 1999 | 25 | 62 | 3.2 | 0.21 | 168 | 1 | 0.33 | 0.56 | NA |
| Auricchio et al, 2003 | 85 | 60 | NA | 0.23 | 155 | 1 | 0.38 | 0.66 | NA |
| Bakker et al, 2000 | 12 | 64 | 3.7 | 0.15 | 194 | 1 | 0.33 | 0.42 | 0.21 |
| Braunschweig et al, 2000 | 16 | 64 | 3.1 | 0.22 | 181 | 0.56 | 0.69 | 0.94 | NA |
| Flevari et al, 2006 | 25 | 66 | 3.2 | 0.27 | 195 | 1 | 0.44 | 0.76 | 0.34 |
| Fung et al, 2008 | 97 | 64 | 3.2 | 0.26 | NA | 1 | 0.42 | 0.74 | 0.34 |
| Inage et al, 2008 | 17 | 63 | 3.2 | 0.25 | 161 | NA | 0.12 | 0.65 | 0.33 |
| Knaapen et al, 2004 | 14 | 58 | 3.1 | 0.25 | 173 | 1 | 0.43 | 0.57 | 0.37 |
| Kubanek et al, 2006 | 43 | 62 | 3.2 | 0.22 | 195 | 0.79 | 0.42 | 0.86 | 0.24 |
| Madaric et al, 2007 | 28 | 67 | 3 | 0.25 | 171 | 1 | 0.5 | 0.82 | 0.28 |
| Mullens et al, 2008 | 19 | 66 | 3.4 | 0.21 | 177 | NA | 0.55 | 0.8 | 0.34 |
| Nelson et al, 2000 | 22 | 59 | NA | 0.2 | 175 | 1 | 0.23 | NA | NA |
| Sogaard et al, 2001 | 22 | 61 | 3.4 | 0.23 | 184 | 1 | 0.56 | 0.88 | 0.23 |
| Vanderheyden et al, 2008 | 10 | 64 | NA | 0.19 | 179 | 1 | 0.4 | 0.8 | 0.34 |
| Waggoner et al, 2006 | 57 | 61 | 3.2 | 0.25 | 180 | 1 | 0.25 | 0.76 | 0.34 |
⁎ For randomized controlled trials data are presented as medical therapy/cardiac resynchronization arms.
| Study | Inclusion Criteria | SBP (mm Hg) | DBP (mm Hg) |
|---|---|---|---|
| Randomized controlled trials ⁎ | |||
| Piccirillo et al, 2006 | Patients with HF with NYHA class ≥III, EF ≤0.35, QRS duration >120 ms, sinus rhythm, and ischemic cause | 109/112 | 69/68 |
| Bristow et al, 2004 | Patients with HF with NYHA class ≥III, EF ≤0.35, QRS duration ≥120 ms, sinus rhythm, PR >150 ms, HF hospitalization in previous year | 112/111 | 64/68 |
| Cleland et al, 2005 | Patients with HF >18 years old with NYHA class ≥III, EF ≤0.35, QRS duration ≥120 ms, sinus rhythm, LVEDD ≥30 mm | 110/110 | 70/70 |
| Nonrandomized cohort studies | |||
| Auricchio et al, 1999 | Patients with HF with NYHA class ≥III, QRS duration ≥120 ms, sinus rhythm, PR ≥150 ms | 90 | 57 |
| Auricchio et al, 2003 | Patients with HF 18–75 years old with NYHA class ≥II, EF ≤0.30, QRS duration ≥120 ms, sinus rhythm, peak V o 2 ≤18 ml/min/kg on maximal exercise | 113 | NA |
| Bakker et al, 2000 | Patients with HF 18–75 years old with NYHA class ≥III, LBBB with QRS duration ≥120 ms, sinus rhythm | NA | NA |
| Braunschweig et al, 2000 | Patients with HF with NYHA class ≥III, EF <0.40, QRS duration >150 ms | 109 | 70 |
| Flevari et al, 2006 | Patients with HF with NYHA class ≥III, EF <0.35, QRS duration >120 ms, sinus rhythm | 110 | 70 |
| Fung et al, 2008 | Standard CRT indication: NYHA class ≥III, EF ≤0.35, QRS duration ≥120 ms | 105 | NA |
| Inage et al, 2008 | Patients with HF with NYHA class ≥III, QRS duration >120 ms | 98 | NA |
| Knaapen et al, 2004 | Patients with HF with NYHA class ≥III, EF <0.35, QRS duration >120 ms, sinus rhythm, LVEDD >55 mm | 114 | 71 |
| Kubanek et al, 2006 | Patients with HF with NYHA class ≥III, EF ≤0.35, QRS duration ≥140 ms, LVEDD >60 mm | 120 | 76 |
| Madaric et al, 2007 | Standard indication for CRT: NYHA class ≥III, EF ≤0.35, QRS duration ≥120 ms, sinus rhythm | 112 | 71 |
| Mullens et al, 2008 | Patients with HF with NYHA class ≥III, EF <0.35, LBBB with QRS duration >120 ms, HR <70 beats/min, sum of dyssynchrony >102 ms, preserved AV conduction | 111 | NA |
| Nelson et al, 2000 | Patients with HF with NYHA class ≥III, EF <0.35, QRS duration ≥140 ms, sinus rhythm | 110 | 72 |
| Sogaard et al, 2001 | Patients with HF with NYHA class ≥III, QRS duration >120 ms, sinus rhythm | 103 | NA |
| Vanderheyden et al, 2008 | Patients with HF with NYHA class ≥III, EF <0.25, LBBB with QRS duration >140 ms, sinus rhythm, total sum of dyssynchrony > 102 ms, HR <70 beats/min | 113 | NA |
| Waggoner et al, 2006 | Patients with HF with NYHA class ≥III, EF ≤0.35, QRS duration >150 ms, sinus rhythm, LVEDD >60 mm | 113 | 67 |
⁎ Randomized controlled trials data are presented as medical therapy/cardiac resynchronization arms.
All nonrandomized cohort studies reported withdrawals or crossovers or had no withdrawal. In 1 study, patients were unaware of their treatment and 1 study documented that it was single blinded but did not specify who was blinded. Thus, these 2 studies were considered at intermediate risk of bias, whereas the rest of the nonrandomized cohort studies were at high risk of bias. Among the randomized controlled trials, based on the 6 parameters suggested by the Cochrane Review, the Cardiac Resynchronization–Heart Failure (CARE-HF) and Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trials were at low risk of bias with low risk in 4 of 6 categories, whereas the third study by Piccirillo et al was at high risk of bias across all categories.
In nonrandomized cohort studies, compared to baseline there was a significant increase in SBP (difference +4.4 mm Hg, 95% confidence interval [CI] +0.8 to +8.0, p = 0.02) after CRT ( Figure 2 ). This was concordant in the randomized controlled trials, where there was a significantly higher SBP (difference +3.9 mm Hg, 95% CI +1.1 to +6.8, p = 0.007) in the CRT group compared to the medical therapy alone group ( Figure 3 ).
In nonrandomized cohort studies, compared to baseline there was no change in DBP (difference +1.0 mm Hg, 95% CI −0.6 to +2.6, p = 0.20) after CRT ( Figure 4 ). Similarly, in the randomized controlled trials, there was no change in DBP (difference +0.5 mm Hg, 95% CI −0.7 to +1.7, p = 0.40) in the CRT group compared to the medical therapy alone group ( Figure 5 ).




