Fig. 8.1
Effect of olmesartan and amlodipine on CFR in patients with arterial hypertension. Adapted from Naya et al. [10]
The favorable effects of antihypertensive drugs on CMD, therefore, likely mainly depend on other mechanisms, including direct effects on vascular smooth muscle cells, an improvement of oxidative state, endothelial function and diastolic function, as well as effects on autonomic nervous system. Accordingly, agents like dihydropiridines might fail to improve CMD due to reflex sympathetic activation, whereas some ß-blockers might have detrimental effects on diastolic relaxation.
Of note, several studies have also clarified how the impairment of CFR in hypertensive patients is largely independent of the presence and degree of LVH [11], suggesting that CMD is mainly a consequence of vascular remodeling or of functional alterations of endothelial cells and/or smooth muscle cells [12, 13] (see Chaps. 2 and 4).
8.1.2 Hypercholesterolemia
Cholesterol-lowering therapy with statins has been shown to improve endothelium-independent coronary microvascular function in patients with high cholesterol levels [14], although with some discrepancies (Fig. 8.2). Notably, despite several studies showing that statins significantly improve endothelium-dependent vasomotion of large conductance coronary arteries, as well as of peripheral arteries, a significant effect on endothelium-dependent CMD, as assessed by acetylcholine intracoronary Doppler measures, has only been demonstrated in a small study with pravastatin [15].
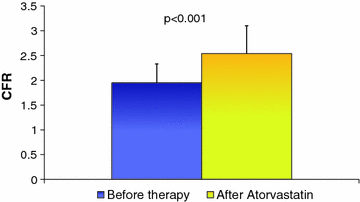

Fig. 8.2
Effect of atorvastatin on CFR in patients with hypercholesterolemia. Adapted from Caliskan et al. [14]
It is still controversial whether the beneficial effects of statins on endothelial function is entirely mediated by the cholesterol lowering effects or also by their pleiotropic, in particular anti-inflammatory, effects.
8.1.3 Diabetes
In contrast with hypertension and hypercholesterolemia, only very few studies investigated the effect of glycemic control on CMD in diabetic patients. A combination of gliburide or glimepiride with metformin was reported to improve CMD in type 2 diabetes mellitus, but contrasting effects were noticed with regard to the relation between glycemic control and the increase of CFR. Acute administration of insulin also was shown to improve CFR in type 1 diabetics, but its long-term effects remain unknown [16] (Fig. 8.3).
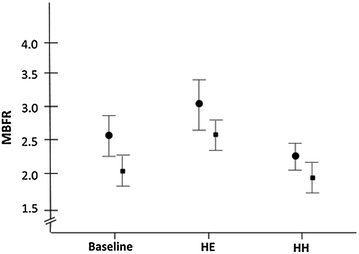

Fig. 8.3
MBF reserve (MBFR; mean ± SD), at baseline and during hyperinsulinemic euglycemia (HE) and hyperinsulinemic hypoglycemia (HH), in type 1 diabetes mellitus patients and in healthy control subjects (●). Adapted from Rana et al. [16]
8.1.4 Other Cardiovascular Risk Factors
Weight loss in obese patients has recently been reported to improve CBF response to dipyridamole, and the improvement was found to correlate with the increase in adiponectin levels [17, 18].
Finally, the reduction of CFR related to smoking has been shown to be normalized by administration of the antioxidant vitamin C, lending support to the notion that the detrimental effects of smoking on coronary microcirculation can be explained, at least in part, by an increased oxidative stress [19].
8.2 Primary Stable MVA
The main goal of treatment of stable MVA is the control of symptoms, as major cardiac events are not increased in these patients [20, 21] (see Chap. 4). The response to treatment, however, is unpredictable, and drug therapy is therefore empirical and requires an optimal interaction between the physician and the patient. Anti-ischemic drugs remain the mainstay form of medical therapy, but their efficacy is limited in several patients. For this reason several alternative forms of therapy have been proposed for unresponsive patients.
Studies assessing the effects of therapy in MVA have several limitations, including the usually small number of patients enrolled, the lack of appropriate controls and the short-term follow-up period.
8.2.1 Classical Anti-ischemic Drugs
Anti-ischemic medications, alone or in combination, constitute the first pharmacological approach to patients with stable MVA [22]. The main results of studies that investigated the effects of classical anti-ischemic drugs in these patients are summarized in Table 8.1.
Table 8.1
Main results of studies assessing the effects of traditional anti-ischaemic drugs in patients with cardiac syndrome X
Drug | Angina | QoL | EST | Holter | NTG use | Other | |
|---|---|---|---|---|---|---|---|
Beta-blockers | |||||||
Fragasso [24] | Atenolol | ↑ | ↑ | Improved diastolic function | |||
Lanza [25] | Atenolol | ↑ | |||||
Leonardo [26] | Atenolol | ↑ | ↑ | Improved diastolic function | |||
Romeo [27] | Acebutolol | ↔ | |||||
Ferrini [28] | Propranolol | ↔ | |||||
Bugiardini [29] | Propranolol | ↑ | |||||
Calcium-antagonists | |||||||
Cannon [30] | Verapamil, Nifedipine | ↑ | ↑ | ↓ | |||
Cannon [30] | Lidoflazine | ↑ | ↑ | ↔ | Severe arrhythmic adverse effects | ||
Ozçelik [31] | Nisoldipine | ↑ | ↑ | ↓ | |||
Romeo [27] | Verapamil | ↔ | |||||
Montorsi [32] | Nifedipine sl | ↑ | ↑ | ↑ CBF | |||
Montorsi [34] | Nifedipine sl | Variable coronary vasomotor response | |||||
Bugiardini [29] | Verapamil | ↔ | |||||
Lanza [25] | Amlodipine | ↔ | |||||
Ferrini [28] | Diltiazem | ↑ | |||||
Sütsch [33] | Diltiazem | ↔ CFR | |||||
Nitrates | |||||||
Lanza [37] | sl ISDN | ↓ | |||||
Radice [38] | sl NTG | ↓ | |||||
Bugiardini [40] | i.c. ISDN | ↓ CBF | |||||
Lanza [25] | ISMN | ↔ | ↔ | ||||
8.2.1.1 Beta-Blockers
ß-blockers may have several beneficial effects in patients with stable MVA. They reduce myocardial oxygen consumption, in particular during exercise and stress conditions, but may also improve coronary perfusion by prolonging diastolic time and improving left ventricular dynamics. On the whole, ß-blockers are particularly useful in patients who show evidence for increased adrenergic activity, including those with increased heart rate at rest, a rapid increase of heart rate and/or blood pressure during exercise, abnormal heart rate circadian rhythm, and sympathovagal imbalance as assessed by heart rate variability [23].
The few studies that assessed the effects of ß-blockers on symptoms in MVA patients have all used atenolol and have all concordantly reported significant beneficial effects, as compared to placebo or other drugs [24–26]. Data are less consistent when exercise test endpoints are considered; indeed, an improvement of ischemic and angina threshold was reported in some studies [24, 26], but not in others [27, 28]. In a study, however, propranolol, but not verapamil, reduced the number of episodes of ST-segment depression during 24-h ECG Holter monitoring [29].
8.2.1.2 Calcium Antagonists
Calcium antagonists are powerful vasodilator drugs and are therefore used in the attempt to improve the reduced vasodilatory capacity of coronary microcirculation; furthermore, they also reduce cardiac afterload. In addition, nondihydropyridine calcium-antagonists reduce myocardial oxygen consumption due to negative chronotropic and inotropic effects. However, calcium-antagonists, in particular dihydropyridine drugs, may rapidly reduce blood pressure, and this effect causes a reflex increase in adrenergic activity, which may antagonise their favorable vasodilatatory effects.
Beneficial effects on chest pain symptoms in MVA have been reported in several studies [27, 30–32]. Furthermore, favorable effects of calcium-antagonists have also been reported on exercise-induced angina and ST-segment depression, as well as on exercise tolerance [28, 30, 32] (Fig. 8.4).
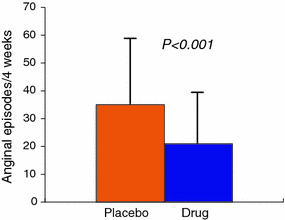

Fig. 8.4
Effect of verapamil or nifedipine (drugs) on episodes of angina in patients with MVA. Adapted from Cannon et al. [30]
However, no significant improvement of angina symptoms were observed with amlodipine in a study [25], and verapamil failed to reduce spontaneous episodes of ST-segment depression during daily life as assessed by ambulatory ECG Holter monitoring in another study [29]. Disappointing results have also reported in the assessment of the effects of calcium antagonists on CBF. No effects of diltiazem on CBF response to dipyridamole were shown in a study [33]; in another study sublingual nifedipine improved myocardial perfusion, but was associated with a worsening of exercise-induced angina and ECG changes in a few patients [34].
In summary, nondihydropyridine calcium antagonists constitute a valid therapeutic first-line option, alternative to ß-blockers, particularly in patients in whom the prevailing mechanism of angina appears to be an increased microvascular constriction, as in patients who also report angina at rest.
8.2.1.3 Nitrates
The anti-ischemic effects of nitrates are mainly related to their capacity to reduce cardiac work through a reduction of preload, although they also have coronary dilator effects. However, the effects of nitrates on the coronary microcirculation seem to be variable and rather limited [35].
As in patients with obstructive CAD and in those with epicardial spam, sublingual short-acting nitrates are the first-choice drugs to treat angina attacks also in patients with MVA. However, the efficacy of sublingual nitrates seems less consistent in these patients, as a complete effect seems to be achieved in only about a half of patients [20, 36].
This is in agreement with the poor, or even negative, effects of short-acting nitrates on ischemic threshold assessed at exercise stress test [37, 38]. Of note, a significant relationship was recently found in MVA patients between the coronary microvascular dilator effect of sublingual nitroglycerin and time to ischemic threshold during exercise test performed after nitrate administration, thus suggesting that it may be beneficial in a subset of patients responsive to its vasodilator effect (Fig. 8.5) [39]. The reasons for the unsatisfactory effects of acute nitrates in MVA patients, in addition to a limited effect on CMD, are not fully known, but may include myocardial hypoperfusion caused by hypotension and reflex adrenergic activation, with an increase in heart rate and, possibly, coronary vasoconstriction. Paradoxical reduction of CBF was indeed observed after intravenous and/or intracoronary short-acting nitrate administration in a study [40].
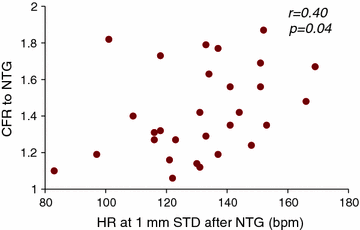

Fig. 8.5
Relationship between heart rate (HR) at 1 mm ST-segment depression (STD) during nitrate exercise stress test (y axis) and coronary blood flow velocity reserve (CFR) to nitroglycerine (NTG) (x axis) in patients with MVA. See text for more detail. Adapted from Russo et al. [39]
There are no data concerning the effects of chronic oral nitrate therapy in CSX patients, with the exception of a small study in which the administration of isosorbide-5-mononitrate (40 mg) failed to improve symptoms and quality of life over a period of 4 weeks [25].
8.2.2 Other Anti-ischemic Drugs
Several other forms of therapy, shown to have anti-ischemic effects, have been assessed in patients with stable MVA and can be prescribed in addition to classical anti-ischemic medications. The main clinical results obtained with these drugs are summarized in Table 8.2.
Table 8.2
Main results of studies assessing the effects of alternative kinds of drugs with potential anti-ischaemic effects in patients with cardiac syndrome X
Drug | Angina | QoL | EST | Holter | NTG use | Other | |
|---|---|---|---|---|---|---|---|
Xanthines | |||||||
Emdin [43] | Aminophylline | ↑ | ↑ | ||||
Yoshio [44] | Aminophylline | ↑ | ↑ | ↑ LVEF at rest but not at peak EST | |||
Radice [38] | Aminophylline | ↑ | ↑ | ||||
Lanza [45] | Aminophylline | ↑ | ↔ | ||||
Lanza [46] | Bamiphylline | ↔ | |||||
Elliott [47] | Aminophylline | ↑ | ↑ | ↔ | |||
ACE-inhibitors | |||||||
Kaski [50] | Enalapril | ↑ | |||||
Nalbantgil [51] | Cilazapril | ↑ | |||||
Ozcelik [31] | Ramipril | ↑ | ↑ | ||||
Pizzi [52] | Ramipril | ↑ | ↑ | ↓ Oxidative stress | |||
Chen [53] | Enalapril | ↑ | ↑ CFR and endothelial function | ||||
Alpha-antagonists | |||||||
Camici [49] | Doxazosin | ↑ | ↑ | ↑CFR | |||
Rosen [54] | Doxazosin | ↔ CFR, chest pain and ECG changes after dipyridamole. | |||||
Bøtker [56] | Doxazosin | ↔ | |||||
Galassi [57] | Prazosin | ↔ | ↔ | ||||
Nicorandil | |||||||
Yamabe [60] | ↑ | ↑ Perfusion defects at scintigraphy | |||||
Chen [61] | ↑ | ↑ | ↔ | ||||
Trimetazidine | |||||||
Leonardo [26] | ↔ | ↔ | ↔ Diastolic function | ||||
Ranolazine | |||||||
Mehta [65] | ↑ | ↑ | ↑ Perfusion defects at CMR | ||||
Villano [66] | ↑ | ↑ | ↑ | ↔ CFR and FMD | |||
Ivabradine | |||||||
Villano [66] | ↑ | ↑ | ↔ | ↔ CFR and FMD | |||
Statins | |||||||
Fábián [69] | Simvastatin | ↑ | ↑ FMD | ||||
Kayikcioglu [70] | Pravastatin | ↑ | ↑ FMD | ||||
Pizzi [52] | Atorvastatin | ↑ | ↑ | ↑ FMD; ↓ oxidative stress | |||
Estrogens | |||||||
Rosano [76] | 17-ß-oestradiol | ↑ | ↑ | ||||
Albertsson [77] | 17-ß-oestradiol | ↑ | ↑ | ||||
8.2.2.1 Xanthine Derivatives
The beneficial effects of xanthines in MVA can be related to two different mechanisms [41]. First, xanthines inhibit the arteriolar dilator effects of adenosine, through antagonism on vascular A2 receptors on smooth muscle cells. This effect in nondysfunctional coronary microvessels may favor CBF redistribution toward myocardial areas with CMD, where the release of adenosine is increased. Second, xanthines stimulate presynaptic α-1 sympathetic receptors; this results in enhanced noradrenaline concentrations in the synaptic space, which, again, causes a more significant microvascular constriction in nondysfunctional areas, thus favoring CBF toward dysfunctional microvessels. Furthermore, xanthines may also have, in addition, an “analgesic” effect, as they antagonize the stimulation of cardiac nerve pain fibers by adenosine, which is a major mediator of ischemic pain [41]. This effect can be particularly important in patients with a reduced pain threshold [42].
A reduction of exercise-induced angina symptoms and/or ischemic ECG changes by acute intravenous administration of aminophylline, a nonspecific A1/A2 adenosine receptor antagonist, in MVA patients was reported in two small controlled trials [43, 44]. A single oral dose of aminophylline (400 mg) [38] was also reported to significantly improve ischemic ST-segment depression and angina pain induced by exercise stress test in a study.
In an uncontrolled trial, however, intravenous aminophylline failed to improve exercise induced angina and ST-segment changes [45], and, in a small randomized placebo-controlled trial, intravenous administration of bamiphylline (300 mg) [46], a specific adenosine A1-receptor antagonist, had no effect on exercise-induced ST segment changes, although reduced the severity of exercise-induced chest pain.
Only one study assessed the effects of chronic oral xanthine therapy on spontaneous angina attacks [47]. In this randomized crossover study 13 MVA patients received either aminophylline (225–350 mg twice daily) or placebo for 3 weeks. In the 10 patients who completed the trial, aminophylline failed to significantly improve angina episodes; furthermore, the drug was found to improve exercise induced angina, but not exercise induced ST-segment change nor episodes of ST-segment depression on ECG Holter monitoring (Fig. 8.6).
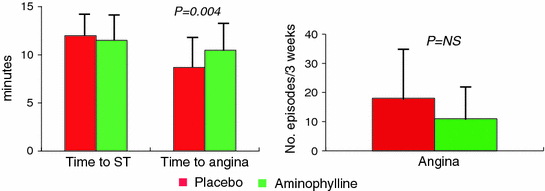

Fig. 8.6
Effect of aminophylline on exercise test (left panel) and on the number of episodes of angina (right panel) in patients with MVA. See text for more detail. Adapted from Elliott et al. [47]
Thus, although data suggest that xanthines may improve exercise-induced angina in MVA patients and pharmacodynamics suggests their utility in case of increased pain sensitivity, their effects on spontaneous angina episodes during continuous therapy need further assessment.
8.2.2.2 ACE-Inhibitors
ACE-inhibitors have been proposed as therapeutic agents in MVA due to their lowering effects of serum and tissue angiotensin II. Although there is no evidence of a major role of angiotensin II in the CMD of these patients, local tissue angiotensin II, in particular, is involved in the regulation of coronary microvascular structure and function, exerting, when increased, several potentially deleterious effects, including vasoconstriction, increased oxidative stress, and degradation of nitric oxide [48]. Furthermore, angiotensin II enhances the effects of sympathetic nervous system on coronary microvascular tone and may also favor structural microvascular changes by stimulating cell growth [48]. Accordingly, enalapril has been found to improve CMD through increase of NO availability and reduction of oxidative stress in MVA patients [49].
The favorable clinical effects of ACE inhibitors in stable MVA were suggested by a small randomized placebo-controlled trial [50], in which enalapril prolonged time to ischemic threshold. Similar results have subsequently been obtained with the use of cilazapril [51]. Moreover, an improvement of angina symptoms, as well as of exercise capacity, has been reported with the use of ramipril [31, 52, 53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


