Fig. 10.1
Therapeutic interventions potentially able to promote collateral vessel growth. Adapted from Chillian et al. [1]
Gene therapy initially appeared as the most applicable and potentially valid form of therapy to stimulate and enhance microcirculation and collateral growth in refractory angina patients. Intracoronary delivery of virus vectors transporting modified genes encoding for angiogenic factors, as vascular endothelial growth factor or basic fibroblast growth factor, has been tested in placebo-controlled trials.
In contrast with findings observed in animal models [1], and after some initial promising data in uncontrolled studies, the results in controlled randomized studies have on the whole been disappointing, as they failed to show a detectable increase of collateral growth in patients, together with no or negligible benefit on symptoms and myocardial ischemia, as compared with control groups (Fig. 10.2) [2–4]. It has been suggested that an increase of oxidative stress might deny the favorable effects of gene therapy. Accordingly, therapy associated with drugs able to restore redox balance is under investigation to establish whether a combined approach has the potential to improve myocardial perfusion. Similar disappointing results have been shown in controlled clinical trials for the direct intracoronary or intramyocardial injection of recombinant angiogenic factors, as vascular endothelial growth factor or basic fibroblast growth factor [5].
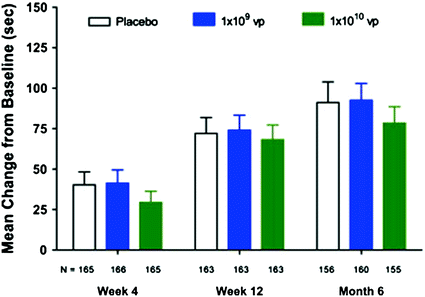

Fig. 10.2
Change in duration of exercise stress test at 4, 12 weeks, and 6 months in patients enrolled in the AGENT-3 and AGENT-4 trials. Data are mean and standard error. vp = viral particles. Adapted from Henry et al. [3]
Attempts to promote collateral circulation in patients with refractory angina have been based also on the intramyocardial administration of progenitor vascular cells (Fig. 10.3) [6–9]. In this case, again, the early clinical results do not allow to establish firm conclusions about the efficacy of this form of therapy, which needs to be addressed in larger studies. Furthermore, in one of these trials some concern derived from the observation of troponin rise during cell mobilization and collection [9].
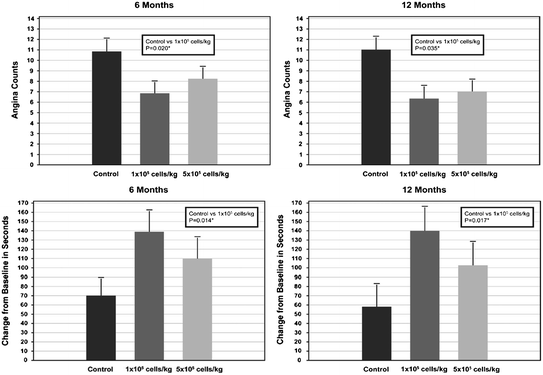

Fig. 10.3
Top panel: Number of anginal episodes per week at 6 and 12 months in patients randomized to intramyocardial injection of placebo or two increasing doses of mobilized autologous CD 34+ cells; both active treatments resulted in a reduction of angina frequency which, however, was statistically significant for the lower dose only. Bottom panel: Change in exercise time at 6 and 12 months after randomization; results are consistent with those for angina frequency. Adapted from Losordo et al. [9]
Exercise is a possible physiologic stimulator of collateral growth, as repetitive increase of myocardial oxygen requirements might activate stimuli for collateral growth; the few studies carried out to this scope, however, have produced controversial results [10–14].
Bioenergetics therapy , e.g., the administration of substances able to improve defective mitochondrial function, such as mitochondrial directed free radical scavengers, have been able to improve collateral circulation in animal models with metabolic syndrome [1], but data in humans are lacking.
Enhanced external counterpulsation has been suggested to stimulate angiogenesis, although the mechanism remains speculative [15]. Clinical observational studies have consistently reported beneficial effects of this form of treatment in patients with refractory angina (Fig. 10.4) [16] and some benefits have been shown in a small randomized sham controlled study [17]. The role of collateral growth stimulation in mediating these beneficial effects, however, remains to be clarified.
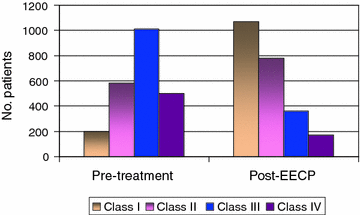

Fig. 10.4
Effect of enhanced external counterpulsation (EECP) on Canadian Cardiovascular Society class in patients with stable angina. Adapted from Wu et al. [16]
Finally, small molecule therapies to target key pathways for stimulation and inhibition are also on the horizon, but are still under investigation [18].
10.2 Treatment of MVO in STEMI
MVO denies the benefits of successful epicardial coronary revascularization in about one-third of STEMI patients, as it is associated with increased morbidity and mortality (see Chap. 6). Several forms of interventions have been proposed in the attempt to prevent or treat MVO, and therefore improve clinical outcome of STEMI patients.
10.2.1 Prevention
Among cardiovascular risk factors, hypercholesterolemia and hyperglycemia, have been shown to be significantly associated with MVO during STEMI [19, 20]; thus, optimal control of these risk factors might provide the advantage, in addition to the other well-known beneficial effects, of preventing MVO in case of STEMI.
Avoidance of substances which contrast preconditioning, like sulfonylureas [21] and high doses of alcohol [22] can be another way to prevent MVO during STEMI. Of note in several studies, sulfonylureas have been found to be associated with a higher rate of death and major cardiovascular events as compared to metformin [23]; although several interpretations can be given for this finding, inhibition of ischemic conditioning by sulfonylureas likely significantly contributes to their detrimental effects.
10.2.2 Mechanical Therapy
Distal embolization of thrombus and debris material during primary PCI has been suggested to play a major role in MVO. This has suggested that the use of devices able to avoid distal embolization might reduce MVO and AMI extension, and improve clinical outcome.
Minimization of balloon inflations with direct stent deployment and no predilatation was first suggested to limit coronary embolization and MVO [24], but results have been inconclusive.
Distal protection devices prevent embolization either by the occlusion of the coronary artery distally to thrombosis with a balloon catheter and the subsequent aspiration of the thrombotic material, or by trapping the embolizing material during PCI in a distal umbrella-like device. A proximal balloon occlusion has also been used with aspiration of debris before balloon deflation (Fig. 10.5) [25].
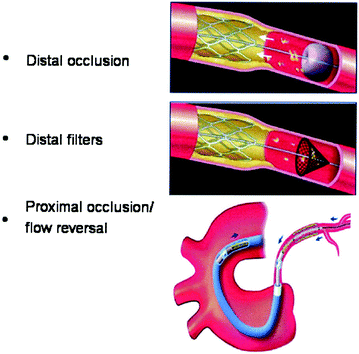

Fig. 10.5
Mechanism of action of devices utilized to prevent distal embolization in STEMI. Adapted from Mauri et al. [25]
Embolic protection devices were initially found effective in the setting of STEMI, showing, in small studies, earlier ST-segment resolution, better TIMI flow and MBG, lower peak enzyme, and improved LV function [26]. Yet, the initial data were not confirmed in larger studies, which failed to show benefit on infarct size and clinical outcome [27]. Meta-analyses [28, 29] have confirmed these disappointing clinical results, in spite of an overall significant improvement of TIMI flow and myocardial blush grade [30].
The lack of success of these devices in acute STEMI may be related to several factors, including prolongation of time to reperfusion, lack of microvascular protection from vasoconstrictor substances, inadequate protection of side branches, embolisation of thrombus material despite the device while crossing the culprit lesion [31].
Direct thrombus aspiration with a dedicated catheter has shown more favorable results. Indeed, two small randomized studies [32, 33] found a beneficial effect of thrombus aspiration on surrogate end-points of myocardial reperfusion. Then, in the TAPAS trial, which randomized 1,071 STEMI patients undergoing primary PCI to thrombus aspiration or control, thrombus aspiration was associated with a significant reduction of major cardiac events (death/re-infarction) at 12 months [34, 35]. This study, however, was characterized by an unusually high rate of deaths in the control group (Fig. 10.6); thus, questioning the applicability of the results. Furthermore, in a recent study, thrombus aspiration failed to reduce infarct size [36].
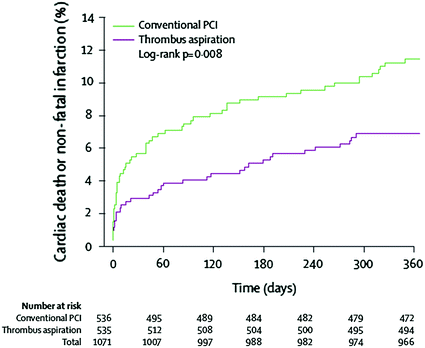

Fig. 10.6
Death rate in the TAPAS trial in patients with STEMI randomized to conventional primary PCI or PCI preceded by manual thrombus aspiration. Death rate was significantly lower in patients randomized to thrombus aspiration. Adapted from Vlaar et al. [34]
Thus, thrombus aspiration seems promising and it is widely applied and recommended by international guidelines [37], but more studies are warranted to definitely prove its real efficacy.
10.2.3 Pharmacological Therapy
10.2.3.1 Antiplatelet Drugs
The use of antiplatelet drugs is based on the central role that platelet activation plays in MVO. The administration of glycoprotein-IIb/IIIa inhibitors, and in particular abciximab, given prior to recanalization, has been shown to improve myocardial perfusion and reduce infarct size in both animal [38] and human studies [39–41], probably through an improvement of MVO. A meta-analysis confirmed that abciximab in patients with STEMI undergoing primary PCI was associated with a lower rate of death and reinfarction up to 3 years follow-up [42], which is consistent with the beneficial effect on clinical outcome shown by abciximab in patients with non-ST segment elevation myocardial infarction [43, 44].
Initial studies suggested that intracoronary administration of abciximab might be more beneficial than intravenous administration [45]. More recent controlled randomized trials, however, have produced conflicting results [46, 47]. Overall, the evidence suggests that platelet glycoprotein-IIb/IIIa antagonists can be helpful in acute STEMI treated by PCI when the intervention is performed on high-risk coronary lesions [48]. Accordingly, current guidelines recommend the use of IIb/IIIa antagonists in patients undergoing primary PCI at high risk due to with a large thrombotic burden [37].
10.2.3.2 Adenosine
Adenosine has been proposed for the prevention of MVO for its multiple potential pharmacological effects, including myocardial conditioning, arteriolar dilatation, inhibition of neutrophil adhesion and migration, platelet activation, and oxygen free radical generation [49].
The AMISTAD trial showed that intravenous adenosine administration, started prior to thrombolysis, was associated with a reduction of infarct size [50], although the benefit was limited to anterior STEMI. Thus, the AMISTAD-II study randomized 2,118 patients with anterior STEMI to intravenous adenosine infusion (50 or 70 μg/kg/min) or placebo prior to thrombolysis or primary PCI [51]. The trial failed to show a benefit of adenosine on clinical outcome, despite a reduction of infarct size with the higher dose (Fig. 10.7). Post-hoc analysis of the AMISTAD-II trial, however, showed that early (<3 h) adenosine administration was associated with a reduction of mortality and major cardiac events at follow-up. Other studies confirmed that adenosine might be beneficial on MVO only when started early (within 4 h) after STEMI onset [52].
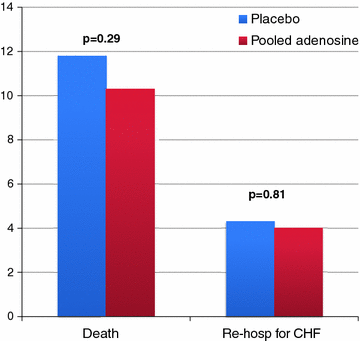

Fig. 10.7
Clinical end-points in STEMI patients treated with adenosine or placebo in the AMISTAD-II trial. Adapted from Ross et al. [51]
Further studies suggested that high doses of intracoronary (rather than intravenous) adenosine can be associated with a lower occurrence of MVO and a more favorable clinical outcome in STEMI [49]. Accordingly, a recent medium-sized controlled randomized trial found that a high dose of intracoronary adenosine, but not nitroprusside, given immediately after thrombus aspiration in patients treated with abciximab, resulted in a lower rate of MVO as compared to placebo; the trial was not powered enough, however, to assess the effects on clinical end-points [53].
In summary, adenosine has shown promising results, in particular with early high-dose intracoronary administration, in the prevention and treatment of MVO; however, further studies are needed to confirm these promising data and to assess whether these favorable effects translate into improvement of clinical outcome.
10.2.3.3 Sodium Nitroprusside
Several studies have assessed the effects of sodium nitroprusside on MVO. This drug is a direct donor of NO, with powerful arteriolar dilatator activity. Excess NO, however, might be detrimental, because of enhanced generation of reactive oxygen species.
Compared to adenosine, in small observational studies intracoronary sodium nitroprusside was associated with a rapid improvement of TIMI flow [54–57].
In another observational study, sodium nitroprusside failed to reduce in-hospital death [57]. Furthermore, in a small randomized, double blind placebo-controlled study [58] intracoronary nitroprusside did not improve TIMI frame count or ST-segment resolution, although its use was associated with a reduction of the composite secondary end-point of target lesion revascularization, re-AMI or death. Finally, in a recent study, adenosine but not nitropruside improved MVO [53].
Thus, intracoronary sodium nitroprusside has the potential to improve myocardial perfusion in patients exhibiting MVO, but the results of clinical studies are rather conflicting.
10.2.3.4 Calcium Channel Blockers
Calcium channel blockers may improve CBF by direct relaxation of SMCs, and by endothelium-mediated vasodilatation; they also may reduce myocardial oxygen consumption by negative inotropic and chronotropic effects, and may reduce oxygen free radical damage during reperfusion [59].
Calcium channel blockers have extensively been investigated in both the prevention and treatment of MVO. In a rat heart model of ischemia-reperfusion, pretreatment with diltiazem, verapamil, or nifedipine improved both endothelium-dependent and endothelium-independent coronary microvascular functions [60]. Furthermore, gallopamil [61] was found to reduce MVO in rabbit hearts.
In STEMI, verapamil was shown to improve TIMI flow when administered before but not after PCI [62]. In other studies, however, intracoronary verapamil improved TIMI flow in patients with established MVO [63, 64].
Thus, nonrandomized/observational studies suggest benefit from intracoronary administration of verapamil also in patients with established MVO. These studies, however, require validation in larger, controlled randomized trials.
10.2.3.5 Nicorandil
Nicorandil is an ATP-sensitive potassium channel opener and NO donor. Studies in animals have demonstrated that nicorandil favors myocardial recovery, reduces infarct size, and possibly decreases neutrophil activation [65].
In several small trials, nicorandil demonstrated benefits in the setting of STEMI [66] with improvement of CBF, regional wall motion and infarct size. In one study [67], nicorandil decreased urinary excretion of the marker of oxidative stress 8-epi-prostaglandin-F2α, with a nonsignificant trend toward a reduction of MVO.
It is possible, therefore, that nicorandil might reduce MVO by reduction of oxygen free radical generation. Again, larger clinical trials are required to validate these promising initial clinical findings.
10.2.3.6 Intracoronary Thrombolysis
Intravenous thrombolysis significantly improved survival in STEMI patients, particularly when administered within 3 h from symptom onset [68, 69]. Intracoronary thrombolysis was hypothesized to prevent MVO in STEMI patients treated by PCI, due to the presumed pathogenetic role of microembolization. However, trials of intracoronary thrombolytics in this setting suggested little or no benefit [70]. A recent randomized study showed, in fact, that intracoronary streptokinase improved CFR [71], but these acute effects were not associated with improved LV function at 6 months, thus questioning the actual role of thrombolysis in this setting.
10.2.4 Ischemic Conditioning
Ischemic preconditioning consists in an increased resistance of myocardial cells to ischemic injury conferred by the exposure to previous short ischemic episodes [72].
More recently, it has been demonstrated that the induction of short episodes of myocardial ischemia during myocardial reperfusion achieved by PCI in the infarct-related occluded artery also portends protection on myocardium, likely reducing reperfusion injury, a phenomenon defined “ischemic post-conditioning” [73, 74]. Similar complex signal pathways seem involved in these two phenomena, including cell-surface receptors, reperfusion injury salvage kinases, free radicals, ATP-dependent potassium channels, and mitochondrial permeability-transition pores [75, 76]. Also nonmyocardial mechanisms might be involved in vivo, including vascular/collateral modulation and platelet reactivity [77].
The role of preconditioning has been confirmed in several clinical studies, in particular during serial balloon inflations during PCI, with reduction of clinical, ECG, hemodynamic, and metabolic evidence of myocardial ischemia [78]. Ischemic preconditioning is likely involved in the reduction of AMI size and improved in-hospital outcome in patients who had preinfarction angina [79].
In the setting of STEMI with PCI, postconditioning by repeated brief episodes of balloon inflation after stenting in the infarct-related artery limited enzyme release with improvement in myocardial blush grade [80], as well as infarct size as assessed by PET and LVEF at echocardiography [81].
The “preconditioning pathway” can be activated also by remote preconditioning. In a recent pilot study [82], remote ischemic preconditioning in patients with evolving STEMI (intermittent arm ischemia through four cycles of 5-min inflations and 5-min deflations of a blood-pressure cuff) significantly improved myocardial salvage as measured by myocardial perfusion imaging (Fig. 10.8). However, these findings need to be confirmed in larger clinical studies.
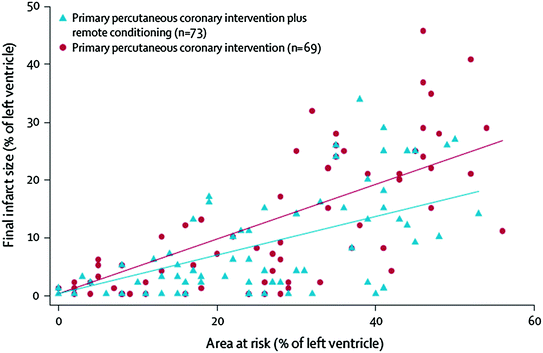

Fig. 10.8
Relation between final infarct size and area at risk for patients receiving primary PCI with or without remote conditioning (per-protocol analysis). The improvement given by remote preconditioning was proportional to the extent of the area at risk. Adapted from Botker et al. [82]
As noted above, preconditioning and postconditioning as well as remote conditioning appear to involve signal-transduction pathways that act on mitochondrial permeability-transition pores [83]. The immunosuppressant cyclosporine also inhibits mitochondrial permeability-transition pore opening. In a small pilot study [84], cyclosporine has recently been shown to reduce infarct size (as measured by CK release and CMR imaging) when given intravenously immediately before primary PCI, with a trend to an improvement of LV end-systolic volume at 6 months [85].
Atrial natriuretic peptide also appears to activate preconditioning; in the J-WIND study patients with STEMI undergoing reperfusion therapy randomized to intravenous administration of atrial natriuretic peptide exhibited lower infarct size, fewer reperfusion injuries, and better outcomes than controls [86].
10.2.5 New Potential Approaches
Other potential approaches have been suggested for the prevention of MVO, including: (1) endothelin-1 receptor antagonists, which induce vasodilatation and also reduce microvascular permeability and the consequent myocardial edema [87]; (2) prostacyclin analogs, which are potent vasodilators and inhibitors of platelet aggregation [88]; and (3) thromboxane A2 receptor antagonists which may mitigate the potent vasoconstrictor and platelet aggregatory properties of thromboxane-A2 [89].
All these drugs have not yet been tested in man, however, and therefore need to be assessed in adequately designed controlled studies.
References
1.
Chilian WM, Penn MS, Pung YF et al (2012) Coronary collateral growth–back to the future. J Mol Cell Cardiol 52:905–911PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


