Transradial Approach
Joseph G. Motwani
The radial artery was first used as an access route for central vascular imaging as far back as 1948 (1). The first transradial coronary arteriogram was performed in 1964, as an extrapolation of Mason Sones’ transbrachial technique, by direct surgical cutdown on to the proximal radial artery just after its origin (2). Subsequent development of percutaneous femoral, axillary, and brachial approaches led finally to the description by Campeau, in 1989, of the percutaneous distal transradial approach for diagnostic coronary angiography (3). In 1993, Kiemeneij et al. extended the applicability of this approach to percutaneous coronary intervention (PCI), a development facilitated by the availability of 6 Fr guiding catheters (4). During the ensuing decade, uptake of the transradial approach has risen gradually, with currently several hundred centers worldwide using this access route for PCI either selectively or (as in my own practice) almost exclusively. The rationale for this increased uptake has two key elements. First, use of the radial artery, a small-caliber, easily compressible vessel, allows virtual elimination of access-site bleeding (5,6), which remains a problem with the transfemoral approach. Even with weight-adjusted heparin dosing, small-caliber guide catheters, and the widespread use of compression devices, significant access-site bleeding occurred in almost 10% of patients in a recent series of transfemoral PCIs performed in association with intravenous platelet glycoprotein IIb/IIIa receptor blockade (7). Femoral closure devices do not reduce appreciably these vascular complications (8). Second, with the rapid expansion of PCI worldwide, now upwards of 2 million procedures annually, inpatient bed availability is becoming an important rate-limiting factor. The transradial approach facilitates earlier patient mobilization and discharge (6,9), including allowing a strategy of day-case PCI in a subset of patients (10).
ANATOMICAL CONSIDERATIONS
The radial artery arises in the antecubital fossa from the bifurcation of the brachial artery into radial and ulnar arteries. The well-recognized proneness of the radial artery to spasm is at least in part attributable to its prominent media. The vessel runs laterally in the forearm to the wrist and, as it is not a component of any neurovascular bundle, unlike the brachial or femoral arteries, there is no risk of nerve trauma during radial artery puncture. Distal to the wrist, the radial artery runs deep to the extensor tendons of the thumb to reach the dorsum of the hand. Finally, it dips between the heads of the interossei to reach the palmar aspect of the hand, uniting with the volar branch of the ulnar artery to create the deep volar arch. The dual blood supply to the hand via the volar arch confers a unique advantage on this vessel as an arterial access route: Unlike the brachial artery, an end-artery, interruption of the radial arterial blood supply will not compromise hand perfusion, providing the collateral arch is intact, as verified by the Allen’s test. This test entails first asking the patient to make a tight fist to exsanguinate the hand, with subsequent digital pressure occlusion by the examiner of the patient’s radial and ulnar arteries. The hand is then relaxed and the ulnar artery is released. Blushing of the hand within 10 seconds confirms an intact collateral supply (Fig. 17.1). The reverse Allen’s test involves the same process, but with release of the radial artery first. The sensitivity of the manual Allen’s test may be improved using digital plethysmography or pulse oximetry to determine the presence or otherwise of an intact waveform at the tip of the middle finger during radial compression. In a recent comparative study, only 1.5% of patients were excluded from use of the transradial approach using these modified techniques, as compared with 6.3% using the manual Allen’s test (11).
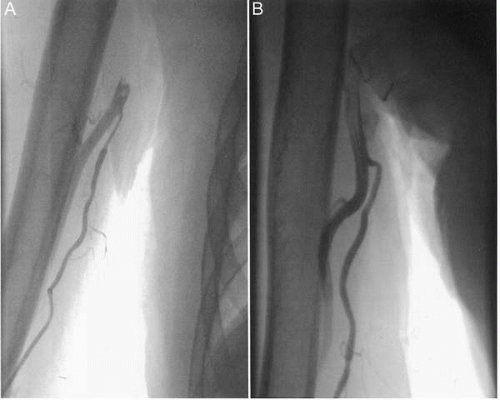 Figure 17.1. High bifurcation of brachial artery in upper arm with radial artery (A) in spasm, and (B) relaxed, post-administration of intra-arterial verapamil. |
Anatomic variations in the radial artery can occur, observed in almost 10% of patients in one series (12), and several of these are of practical relevance. First, the radial artery may have a high origin in the upper arm, from axillary or brachial arteries. This may cause difficulty in catheter manipulation, and it is associated with more frequent spasm (Fig. 17.2). Second, shortly after its origin in the antecubital fossa, the radial artery gives rise to a recurrent branch that courses back to supply the elbow joint and anastomoses with the profunda branch of the brachial artery. If a large radial recurrent branch is cannulated, not only will the catheter not pass beyond the upper arm, but recognition of this problem is necessary to avoid vessel perforation and extravasation: careful withdrawal from the radial recurrent branch and redirection of the catheter into the true brachial artery is required. Third, tortuosity is encountered not infrequently in radial or brachial arteries, including occasionally, 360° loops that can impede catheter passage. Specific maneuvers, such as switching from a 0.035-inch diameter J wire to a hydrophilic Terumo wire or even a 0.014-inch angioplasty wire, if necessary supported by a 4 or 5 Fr Judkins right 4 diagnostic catheter, can be useful in negotiating this problem.
RADIAL CANNULATION TECHNIQUE
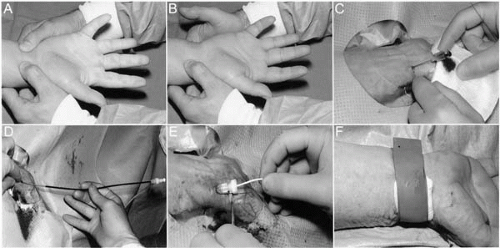
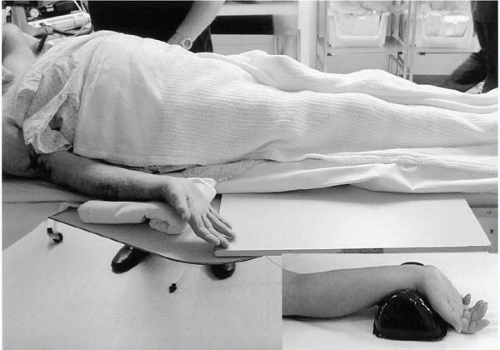
Bracelets, watches, and rings are removed and the wrist and distal 15 cm of the forearm are shaved. The wrist and whole forearm, including the antecubital fossa (in case brachial access is required), are disinfected, and one should avoid siting intravenous cannulae ipsilaterally. Ideally, the right groin also should be prepared in case of transradial access failure or requirement for intra-aortic balloon pump. The supine patient’s arm is extended and slightly abducted, and the wrist hyperextended over a rolled sheet or molded plastic support (Fig. 17.3). The operator palpates the course of the radial artery with the index, middle, and third fingers to identify the point of maximum pulsation at the wrist, usually approximately 1 cm proximal to the radial styloid. The skin over this point is infiltrated with 1 to 2 mL of 1% lidocaine. The radial artery is punctured at a 30° angle with a 21-gauge 4.0 cm-long, bare needle. This is preferable to a cannula-over-needle apparatus because one can more reliably ascertain with a bare needle that the tip is sitting freely in the vessel lumen. Once a steady backflow of blood is attained, either pulsatile flow or rapid dripping, an 0.018-inch diameter nitinol guidewire is advanced through the needle into the vessel. Having removed the needle and made a small linear incision with a scalpel at the wire entry point, a radial introducer sheath with hydrophilic coating and prominently tapered dilator is advanced over the wire into the vessel. Finally, the wire and dilator are removed and, if required, the sheath can be secured in place with a skin suture (Fig. 17.2C-E). Although some operators prefer long (23 to 24 cm) sheaths, because they protect the whole
length of the radial artery up to the bifurcation from direct contact with the torquing guide catheter, in practice shorter sheaths are not associated with any greater frequency of radial spasm, and I now use as standard an 11 cm-long sheath for 6 Fr procedures and a 5.5 cm sheath for 7 Fr and 8 Fr procedures. Following insertion, many operators routinely administer into the sheath a vasodilator cocktail of nitrate 2 to 5 mg and/or verapamil 2 to 5 mg to minimize the incidence of spasm. However, with increasing operator experience, this is not essential. The administration of weight-adjusted heparin forms a standard part of any PCI procedure for most operators, but even if it does not, heparin 5,000 units should be administered for every transradial procedure, either intravenously or via the arterial sheath, to further reduce the low rate of asymptomatic radial artery occlusion that occurs in the weeks following the procedure.
length of the radial artery up to the bifurcation from direct contact with the torquing guide catheter, in practice shorter sheaths are not associated with any greater frequency of radial spasm, and I now use as standard an 11 cm-long sheath for 6 Fr procedures and a 5.5 cm sheath for 7 Fr and 8 Fr procedures. Following insertion, many operators routinely administer into the sheath a vasodilator cocktail of nitrate 2 to 5 mg and/or verapamil 2 to 5 mg to minimize the incidence of spasm. However, with increasing operator experience, this is not essential. The administration of weight-adjusted heparin forms a standard part of any PCI procedure for most operators, but even if it does not, heparin 5,000 units should be administered for every transradial procedure, either intravenously or via the arterial sheath, to further reduce the low rate of asymptomatic radial artery occlusion that occurs in the weeks following the procedure.
GUIDE CATHETER SELECTION
Femoral guide catheters are very adaptable to transradial use (13). Judkins left and right guide catheters can be used for left and right coronary engagement, respectively, remembering that for the left coronary, downsizing to a JL3.5 rather than a JL4 is necessary in the majority of patients. Multipurpose catheters also can be used for both vessels; for the right coronary, Amplatz right and Williams catheters can be useful. If additional support is required for the left coronary, this can be provided by extra backup curves (EBU, XB, GL, Voda), and Amplatz left guides are effective as support guides for both left and right coronaries via the transradial approach. Notable also is that the Judkins left 3.5 guide engages in approximately a third of right coronary ostia, and when it does so, it gives excellent support.
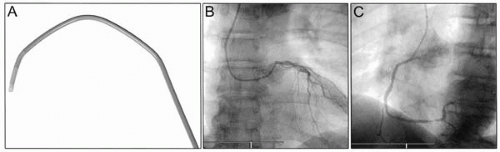 Figure 17.4. Kimny transradial guide catheter (A), engaged in left coronary artery (B), and engaged in right coronary artery (C). |
A range of specific, eponymous “radial” guide catheters— Kimny, Barbeau, Fajadet, Ikari—have been designed to give optimum support and alignment. Although not essential, these can be useful in specific circumstances (14). Several of these specific transradial guide catheters that can be very useful not only in giving excellent support but also in allowing treatment of both left and right coronary arteries with a single guide, for example the Kimny guide (Fig. 17.4) and the “radial” Mach 1 guide (Fig. 17.5).
Regardless of which guide catheter is selected, it should be advanced up the radial and brachial arteries with minimal friction, and all catheter exchanges should be performed over a 0.035-inch diameter exchange length J wire. If any resistance is encountered during passage, dilute contrast should be injected via the guide to identify the problem (e.g., loop, spasm, cannulation of side-branch). The J wire, or if necessary, a Terumo wire or angioplasty wire, followed by the guide, then can be directed up the arm under fluoroscopic guidance.
On reaching the subclavian artery, progression of the wire into the ascending aorta should be followed in the anteroposterior projection, advancing the tip fully down to the aortic valve. For left coronary engagement, the JL3.5 or other guide should be advanced into the aortic root below the left coronary origin. The wire then is removed, the catheter is withdrawn slightly and rotated counterclockwise to achieve engagement. Occasionally, deep inspiration by the patient facilitates this maneuver. For right coronary engagement, it is usually less important to advance the catheter fully into the aortic root before wire removal, the cannulation technique for this vessel being similar to the transfemoral technique.
Sometimes, because of brachiocephalic tortuosity or aortic unfolding, commonly seen in elderly patients, the
guidewire preferentially advances into the descending rather than ascending aorta. In these instances, a specific sequence of maneuvers is usually successful in redirecting into the ascending aorta:
guidewire preferentially advances into the descending rather than ascending aorta. In these instances, a specific sequence of maneuvers is usually successful in redirecting into the ascending aorta:
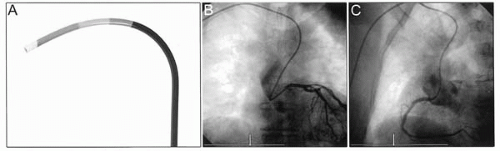 Figure 17.5. Radial Mach 1 guide catheter (A), engaged in left coronary artery (B), and engaged in right coronary artery (C). |
Advance wire and then guide into descending aorta.
Withdraw wire into guide.
Withdraw guide catheter to aortic arch and then rotate it counterclockwise.
Advance wire and then guide into ascending aorta.
Engagement of vein-graft ostia via the transradial approach can be achieved using Judkins right 4, Multipurpose, or bypass graft guide catheters. If difficulties are encountered with these catheters, either failure to cannulate the graft or lack of support, Amplatz left guides are very useful in countering these problems. The left and right transradial approaches are ideally suited to engagement of, respectively, left and right internal mammary grafts, using either internal mammary or Judkins right guide catheters.
POSTPROCEDURE MANAGEMENT
On completion of the procedure, the radial sheath should be removed immediately. If significant discomfort or spasm is encountered during sheath removal, intra-arterial nitrates or verapamil via the sheath, analgesia, sedation, or additional local anaesthetic can be administered. However, since the advent of hydrophilic sheaths, this step is rarely problematic (15). Radial compression and hemostasis can be achieved in a number of ways. At its simplest, a rolled gauze swab and tourniquet (Fig. 17.2F), or compression bandage with double adhesive tape, can be used; both methods are inexpensive but very effective. Alternatively, a range of compression devices are available—RadiStop, Adapty, Hemoband, Radstat—all of which are designed to achieve hemostasis with additional wrist support and avoidance of ulnar artery compression. Regardless of which method is selected, compression should be maintained until hemostasis is attained, a minimum of 1 hour post-PCI, and sometimes considerably longer (for example, if a IIb/IIIa inhibitor has been administered). As part of routine aftercare of the transradial PCI patient, hand perfusion should be monitored for a minimum of 1 to 2 hours postprocedure, using finger pulse oximetry, if preferred. Following an uncomplicated transradial PCI, the patient can sit up immediately and can ambulate after 1 to several hours.
CONTRAINDICATIONS TO USE OF TRANSRADIAL APPROACH
Few contraindications exist to the use of the transradial approach. Absolute contraindications are absence of radial arterial pulse, ischemic Allen’s test, ipsilateral renal dialysis fistula, and requirement for guide size larger than radial artery diameter. Relative contraindications include known atherosclerosis or severe tortuosity of brachiocephalic or subclavian arteries, Raynaud’s disease, and planned internal mammary graft PCI where the ipsilateral transradial approach cannot be used. Collectively, these contraindications exclude less than 5% of patients.
LEARNING CURVE
Unquestionably, a learning curve exists to adopting, or switching to, the transradial approach. Compared with the transfemoral approach, transradial procedures are technically more demanding, at least initially, because of: (a) greater difficulty in cannulating the comparatively small radial artery; (b) variations in radial anatomy, as described earlier; (c) the occurrence of spasm; and occasionally, (d) technical difficulty in cannulating the coronary ostia (16). In practice, for the experienced transfemoral interventionist, the learning curve should be fairly steep. However, it is important to adopt a stepwise approach, which I define as follows:
Stage 1. Perform diagnostic angiography transradially. This enables one, in a nontherapeutic setting, to gain experience in two key aspects—radial artery puncture and transradial catheter manipulation.
Stage 2. Perform selected single-vessel PCIs transradially. Choose stable patients, less than 70 years old, preferably male, with normal left ventricular function, normal aortic root size, and large, easily palpable radial arteries.
Stage 3. Progressive extrapolation to performing unselected PCIs transradially.
I recommend advancing to each subsequent stage when a number of key, objectively defined parameters for the current stage—total procedure time, fluoroscopy time, contrast volume, catheter usage—are close or equivalent to one’s own transfemoral “reference values.”
ARTERIAL SPASM
Although the advent of hydrophilic introducer sheaths has reduced greatly the occurrence of radial artery spasm (15), it remains the commonest complication associated with transradial procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree