TRANSPOSITION OF THE GREAT ARTERIES
Introduction
In the preceding chapters, we addressed wide-ranging topics in a more general fashion. In this and in subsequent chapters, we will discuss more commonly encountered individual cardiac defects.
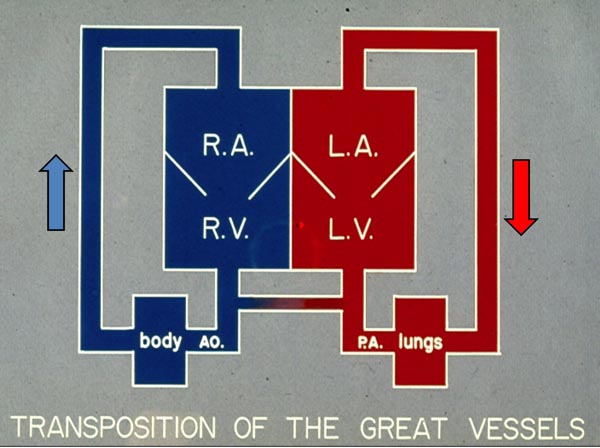
Transposition of the great arteries (TGA) constitutes 5% of all cyanotic congenital heart defects (CHDs) and 10% of all neonatal cyanotic CHDs1 and is the most common cyanotic CHD in the neonate. A variety of descriptions have been used to characterize TGA, but the most precise portrayal is “a CHD in which the morphologic right ventricle (RV) gives rise to the aorta (Ao) and the morphologic left ventricle (LV) gives rise to the pulmonary artery (PA).” In the most frequent type, namely, complete transposition, there is atrial situs solitus (atria are normal in position), atrio-ventricular concordance (right atrium (RA) connected to the RV and the left atrium (LA) to the LV) is present, d-loop of the ventricles (RV is on the right and LV on the left) exists, ventriculo-arterial discordance (Ao arises from the RV and the PA from the LV) occurs, and the aortic valve is positioned to the right of the pulmonary valve (PV) (d-TGA). Therefore, the systemic venous return from the vena cavae (VC) goes into the RA and RV and from there into the Ao, whereas the pulmonary venous blood comes into the LA and LV and from there into the PA (Figure 28.1). Consequently, the circulation is parallel instead of the normal in-series circulation. This results in not delivering the pulmonary venous blood to the body and not getting the systemic venous blood to the lungs for oxygenation. With this state of circulation, the newborn will not survive unless there is intercirculatory mixing via defects such as patent foramen ovale/atrial septal defect (PFO)/(ASD), patent ductus arteriosus (PDA), or ventricular septal defect (VSD).
Figure 28.1. Box diagram of the heart showing parallel circulations in TGA. Note that RV pumps into the Ao. (because of transposition) which goes to the body and returns into RA and back into the body. Similarly, LV output goes to the pulmonary artery (PA and lungs and returns back to the LAand LV to be pumped back into the lungs. Unless there are intercirculatory communications via either a PFO or PDA, the infant cannot survive. Mixing across a VSD if such is present (not shown in the diagram), would also prevent progressive hypoxemia and death. Reproduced with permission from Neonatology Today 2010;5(8):1–7.
Classification
TGA may be classified as Group I, TGA with intact ventricular septum; Group II, TGA with VSD; and Group III, TGA with VSD and pulmonary stenosis (PS).
Clinical Features
Clinical presentation is largely determined by the anatomic type.
Symptoms
The neonates in Group I, TGA with intact ventricular septum frequently present with cyanosis within hours to days of life (usually in the first week of life). They may be asymptomatic otherwise. Yet, with time, they are likely to develop tachypnea and respiratory distress. If no appropriate treatment is provided, these neonates will become acidotic and go on to develop lethargy with lack of spontaneous movement, and may eventually expire.
The babies in Group II, TGA with VSD usually present with symptoms of congestive heart failure (CHF), namely, tachypnea, tachycardia, sweating, and poor feeding. Such presentation is slightly later than that seen in babies with intact ventricular septum, sometime between 2 and 6 weeks of life. However, they are only minimally cyanotic.
The infants in Group III, that is, TGA with VSD and PS, do have a variable presentation; this is dependent upon the extent of intercirculatory mixing and the severity of pulmonary outflow tract obstruction. If poor mixing is present the babies will present early in life and mimic Group I TGA patients with an intact ventricular septum. If the pulmonary outflow tract obstruction is severe, the presentation is basically akin to that seen with tetralogy of Fallot (TOF).2 When PS is moderate the presentation is likely to be late. When PS is mild, signs of CHF may develop in a manner similar to that seen in Group II babies with TGA with VSD.
Physical Examination
The Group I TGA babies with intact ventricular septum typically have severe cyanosis. However, they are not usually in distress until severe hypoxemia and acidosis ensue. Similarly, clubbing is not seen in the neonatal period and may not be seen until 3 to 6 months of age. Increased RV impulse and single second heart sound are present. No cardiac murmurs are usually heard in neonates with intact ventricular septum. Rarely, a grade I to II/VI nonspecific ejection systolic murmur may be auscultated along the left sternal border.
In Group II, TGA babies with VSD, tachypnea, and tachycardia are usually seen. They are minimally cyanotic and have hepatomegaly. Increased RV and LV impulses, single second sound, a grade III to IV/VI holosystolic murmur at the left lower sternal border are the usual findings. In babies with a large ventricular shunt a mid-diastolic flow murmur may be heard at apex.
In Group III, TGA babies with VSD and PS, the physical examination is similar to TGA with intact septum, TGA with VSD, or TOF depending upon the degree of mixing and severity of pulmonary outflow tract obstruction. However, grade III to IV/VI long ejection systolic murmur at the left upper sternal border and/or a holosystolic murmur at the left lower sternal border are usually heard in most of these babies.
Noninvasive Evaluation
Chest x-ray
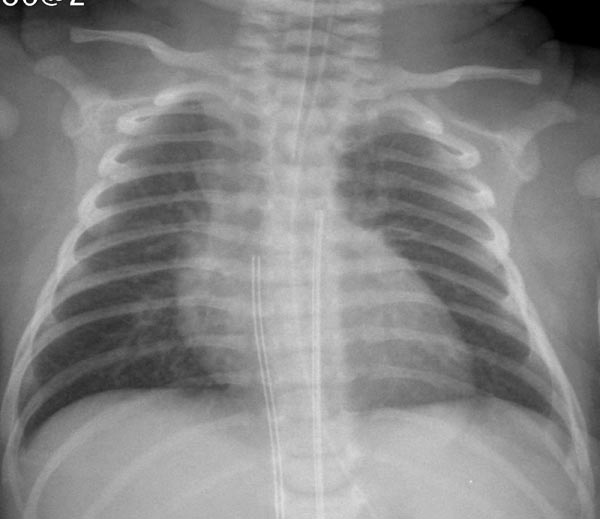
Chest roentgenogram in Group I TGA neonates with intact ventricular septum appears benign with normal to minimal cardiac enlargement and normal to slightly increased pulmonary vascular markings (Figure 28.2). The thymic shadow rapidly involutes giving the appearance of a narrow pedicle (superior mediastinum). Amalgamation of the above signs may at times have the appearance of an “egg-shaped” heart on a posteroanterior chest x-ray. In Group II TGA babies with VSD, moderate-to-severe cardiac enlargement, and increased pulmonary vascular markings are typically visualized. In Group III TGA babies with VSD and PS, mild-to-moderate cardiac enlargement may be noted. The pulmonary vascular marking may be decreased, normal, or increased; this is largely determined by the severity of PS.
Figure 28.2. Chest radiograph of a 2-day old infant with TGA demonstrates mildly enlarged size of the heart and increased pulmonary vascular markings.
ECG
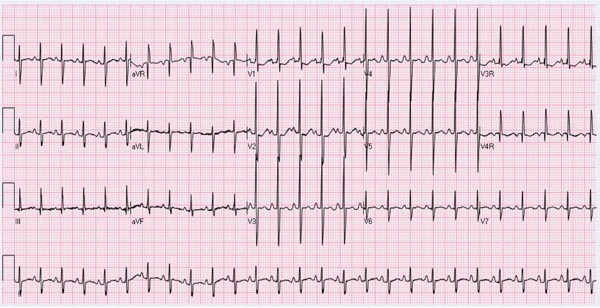
The ECG of the newborn in Group I (TGA and intact ventricular septum) may appear normal with the usual RV preponderance seen at this age (Figure 28.3). However, definitive RV hypertrophy becomes evident in older infants and in children. In addition, RA enlargement may be seen. In babies with Group II (TGA with VSD), biventricular hypertrophy and LA enlargement are usually observed. In newborn babies with Group III (TGA with VSD and PS), RV or biventricular hypertrophy, depending upon the degree of PS may be seen.
Figure 28.3. ECG of a neonate with TGA with intact ventricular septum demonstrating right-axis deviation and prominent R waves in right chest leads and increased S waves in left chest leads suggestive of RV hypertrophy.
Echocardiogram
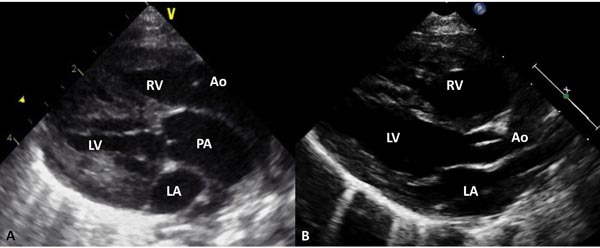
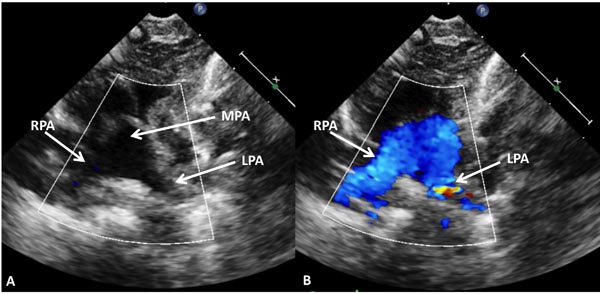
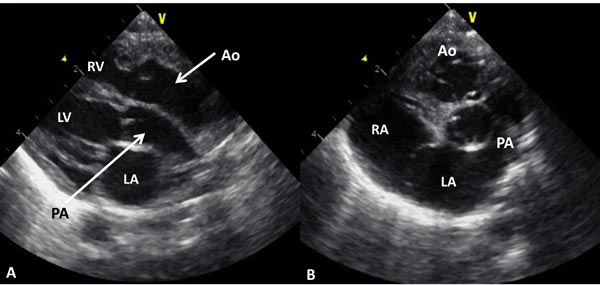
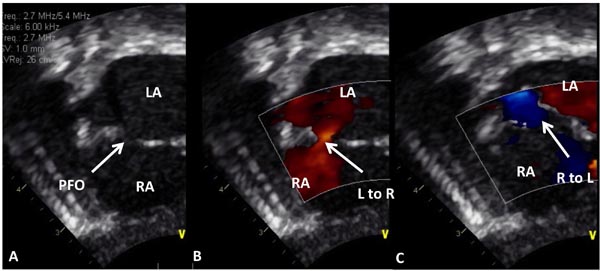
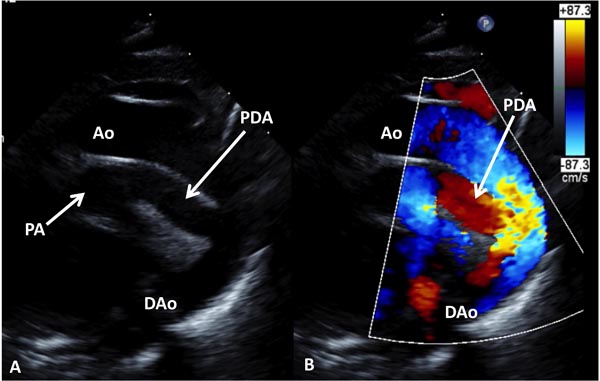
Echocardiogram is useful both in confirming the diagnosis and in the assessment of associated abnormalities. Because of the fact that atrial and ventricular anatomy is normal, as are the aortic and PVs, the diagnosis of TGA may be somewhat difficult by echo, particularly for the neophyte. An indirect sign of somewhat a posterior course of the great vessel coming off of the LV in a precordial long-axis view (Figure 28.4A), indicating that this vessel is a PA, is helpful. This is in contradistinction to an anteriorly coursing ascending Ao (Figure 28.4B) in normal newborns. When the great vessel arising from, the LV is traced and its bifurcation demonstrated (Figure 28.5), that vessel is identified as a PA and the diagnosis of TGA is confirmed. Other helpful signs suggestive of TGA are parallel position of both great arteries in a long-axis view of the heart (Figures 28.4A and 28.6A) and simultaneous on-end visualization of both the Ao and PA on a precordial short-axis view (Figure 28.6B). The atrial septum should be evaluated in the subcostal views both by 2-dimensional (2D) imaging (Figure 28.7A) and color flow mapping (Figure 28.7B and C); the interatrial communication is usually a PFO. Occasionally, a true ASD is present. An interatrial defect of 5 mm diameter or larger is generally considered adequate for providing sufficient interatrial mixing to avoid balloon atrial septostomy. PDA may also be present and the shunt across it by color and pulsed Doppler should be assessed (Figures 28.8 and 28.9).
Figure 28.4. Precordial long-axis echocardiographic views of 2 neonates, first, A: with TGA and second, B: with normally related great arteries. A. Note that the posterior vessel arising from the LV is coursing backward (posteriorly) after its origin from the LV and is likely to be the PA, suggesting TGA. This vessel should be traced and its bifurcation demonstrated (see Figure 11.38) to confirm that it is indeed PA. In B, the posterior vessel arising from the LV courses somewhat anteriorly, indicating that it is likely to be the Ao. Ao, aorta; LA, left atrium; RV, right ventricle.
Figure 28.5. The vessel arising from the LV of the infant shown in Figure 28.4 was traced which showed bifurcation in 2D (A) and color flow (B) images into right (RPA) and left (LPA) pulmonary arteries suggesting that this vessel is main pulmonary artery (MPA), confirming the diagnosis of TGA.
Figure 28.6. Selected video frames from a 2D echocardiogram in long- (A) and short- (B) axis views of an infant with TGA. Note parallel position (A) of the PA and Ao and on-end visualization of PA and Ao in B. These echoes are highly suggestive of TGA; in a normal baby, because of normal criss-crossing of the PA and Ao such appearance is unlikely. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 28.7. Selected video frames from subcostal views of a neonate with TGA demonstrating PFO in A with left to right (L–R) shunt in (B) and right to left (R–L) shunt in C in different phases of the cardiac cycle. LA, left atrium; RA, right atrium.
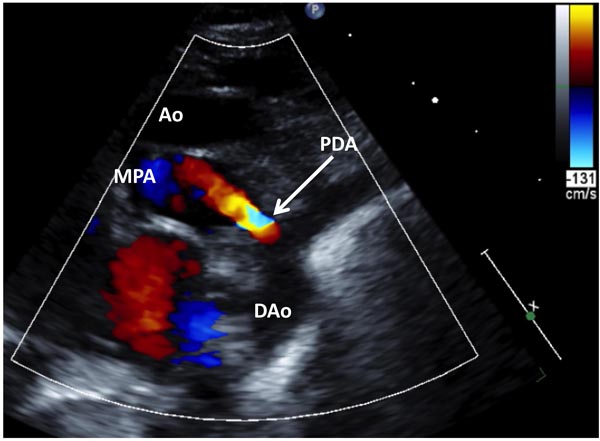
Figure 28.8. Selected video frames from suprasternal notch views of the aortic (Ao) arch of a neonate with TGA demonstrating a large PDA; 2D (A) and color flow images (B) are shown. DAo, descending aorta; PA, pulmonary artery.
Figure 28.9. Selected video frame from suprasternal notch views of the aortic (Ao) arch of another neonate with TGA demonstrating a small PDA. DAo, descending aorta; MPA, main pulmonary artery.
Furthermore, visualization of VSD and PS will help categorization of the babies into the respective groups. Shunting across the VSD by color Doppler (Figure 28.10) and degree of obstruction across the pulmonary outflow tract (Figure 28.11) by pulsed and continuous (or high-frequency pulsed) Doppler (not shown) should be evaluated and these are helpful in assessing physiological state of the baby.
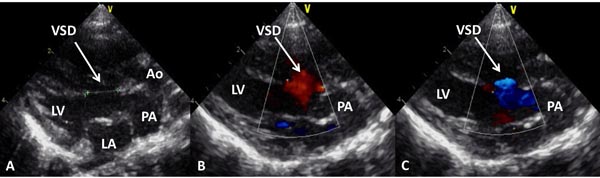
Figure 28.10. Selected video frames from precordial long-axis views of a neonate with TGA demonstrating a large (A) VSD with bidirectional shunting (B and C). Ao, aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery.
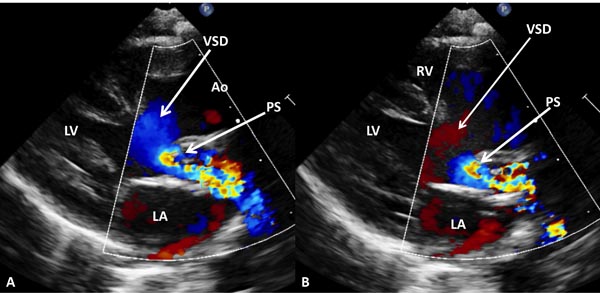
Figure 28.11. Selected video frames from precordial long-axis views of a neonate with TGA demonstrating a large VSD and severe PS. Right to- eft (A) and left to right (B) shunt across the VSD are shown. Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
Other Laboratory Studies
Pulse oxymetry is valuable in identifying the cyanotic infant.3 However, blood gas values are more useful in indicating the extent of hypoxemia and ventilatory status. Serum glucose (or dextrostix), calcium, hemoglobin, and hematocrit values are also helpful in the overall assessment of the baby as reviewed elsewhere4 and are discussed in Chapter 13.
Cardiac Catheterization and Angiography
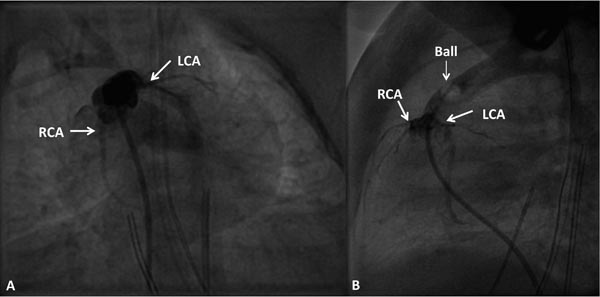
Because of high accuracy of echocardiography in confirming the diagnosis of TGA, invasive catheterization studies are not required for the diagnostic purposes. However, necessity for speedy relief of hypoxemia and acidosis by balloon atrial septostomy, and on occasion, the need for a better definition of coronary arterial anatomy (Figure 28.12) preceding arterial switch operation, may call for catheterization and angiography.
Figure 28.12. Selected cineangiographic frames in caudal (A) and straight lateral (B) views to demonstrate coronary artery anatomy by balloon (Ball) occlusion aortography. Note the normal origins of left (LCA) and right (RCA) coronary arteries.
In Group I (TGA with intact ventricular septum) babies, vena caval, RA, RV, and aortic oxygen saturations are moderate to severely diminished unless atrial, ventricular, or ductal shunting exists. Likewise, the pulmonary venous, LA, LV, and pulmonary arterial saturations are in the high 90s with negligible right-to-left shunt. In babies with transposed great vessels oxygen saturations are higher in the PA than those in the Ao, whereas in babies with normally related great arteries the aortic saturation is higher than that in the PA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree