18 Transposition of the Great Arteries
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
2. The four-chamber view is normal, with equal-sized ventricles and a normal cardiac axis, position, and size (cardiothoracic ratio = 0.3).
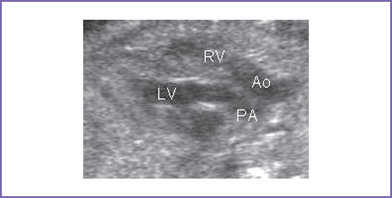
3. The outflow assessment reveals transposition of the great arteries (TGA) (aorta anterior and to the right of the pulmonary artery), with mild asymmetry. Aorta–to–pulmonary artery diameter ratio is 1:0.7 (Fig. 18-1).
4. The pulmonary valve is bicuspid and domes mildly in systole. There is moderate flow acceleration through it by Doppler (1.5 m/s).
5. There are good-sized branch pulmonary arteries. The right pulmonary artery is 2.2 mm and the left pulmonary artery is 2.2 mm.
6. The aortic annulus is of normal size and there is normal Doppler velocity through it.
7. The aortic arch is leftward and the ductal and aortic arches are of similar size. Both arches have antegrade flow.
8. There is a large subpulmonary perimembranous VSD.
9. Flow through the foramen ovale is unrestricted, with a bidirectional shunt.
10. The pulmonary venous flow pattern is normal.
11. The RV and LV Tei indices (myocardial performance index) are normal.
D. Fetal management and counseling
1. Amniocentesis was not offered. The risks from amniocentesis are probably higher than the risk of abnormal karyotype in simple transposition.
2. Follow-up includes serial antenatal studies at 4- to 6-week intervals.
a. The size of the ventricles should be compared. RV hypoplasia is a rare finding but one that can alter the surgical management to a single-ventricle repair.
b. Pulmonary outflow should be monitored for progressive pulmonary outflow tract obstruction as well as progressive main and branch pulmonary arteries and change in direction of ductus arteriosus flow.
c. Progressive foramen ovale restriction can occur (Maeno et al, 1999).
d. Progressive ductus arteriosus constriction can occur (Maeno et al, 1999).
E. Delivery
1. In TGA with pulmonary stenosis, delivery should be at term or as close as possible to term in a tertiary care center given the risk of foramen ovale restriction and ductus arteriosus constriction.
2. In addition, the true degree of pulmonary stenosis after birth and after ductus arteriosus closure may be difficult to fully predict.
F. Neonatal management
a. Prostaglandin E1 (PGE1) infusion to keep the ductus open to increase pulmonary blood flow and raise arterial oxygen saturation.
b. Administration of oxygen can increase oxygen saturation by decreasing the pulmonary vascular resistance and increasing blood flow.
c. Therapeutic balloon atrial septostomy may be performed if the infant is very cyanotic, particularly with metabolic acidosis (a PaO2 level of 25 mm Hg and a pH of less than 7.15).
d. At times, volume and inotropic support may be indicated to improve ventricular function and atrial-level mixing.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis is required for life.
2. If there are residual hemodynamic abnormalities, anticongestive medications and some restriction of activities may be required.
3. The patient should have one or two yearly outpatient follow-up visits with 12-lead electrocardiogram (ECG) and echocardiogram.
4. If there is any history of unexplained palpitations or abnormal heart rhythm, a metabolic stress test with 24-hour Holter monitoring is recommended.
H. Risk of recurrence
Because there is a previous sibling with VSD, the recurrence risk is 3% to 5%.
I. Outcome of this case
1. The baby was born at term with good Apgar scores and good weight.
2. Moderate cyanosis was noted at birth. Pulse oximeter reading was 77%.
3. Postnatal echocardiography confirmed the diagnosis with large subpulmonary VSD with posterior deviation of the outlet septum causing valvar and subvalvar pulmonary stenosis.
4. The pulmonary arteries were of good size, with a transpulmonary Doppler velocity of 4.5 m/s.
5. The foramen ovale and ductus arteriosus were good sized and patent, with bidirectional shunting. PGE1 infusion was started.
6. The baby had a palliative aortopulmonary shunt at 1 week of age followed by a successful Rastelli operation at 12 months of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree