8 Transposition of the Great Arteries
d-TGA: Preoperative Imaging
Background
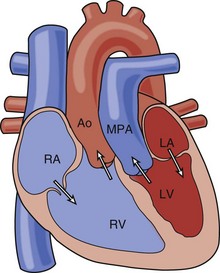
• In this lesion, the atria and ventricles are aligned normally, but the great arteries are transposed, with the aorta (Ao) arising from the right ventricle (RV) and the pulmonary artery (PA) arising from the left ventricle (LV) (Fig. 8-1).
• Deoxygenated systemic venous blood circulates to the right atrium (RA) to the RV to the aorta and thus back to the body.
• Oxygenated pulmonary venous blood circulates to the left atrium (LA) to the LV to the PA and thus back to the lungs.
• Mixing between the two circuits is required to sustain life. This can occur via an atrial septal defect (ASD), ventricular septal defect (VSD), or patent ductus arteriosus (PDA).
• Mixing is generally most effective and reliable at the atrial level. Thus, assessment of the size of the ASD is key for acute management.
Overview of Echocardiographic Approach
• Presence or absence and size of shunts at the atrial, ventricular, and ductal levels (2D, color Doppler).
• Relative size, morphology, and function of the AV and pulmV (2D, color Doppler, pulsed wave [PW] Doppler, continuous wave [CW] Doppler).
Anatomic Imaging
Acquisition
• Subcostal frontal (long axis [LAX]) sweep, posterior to anterior.
• Establish normal visceroatrial situs: visualize the inferior vena cava within the liver on the right, connecting to the right-sided atrium. The left-sided atrium should have some pulmonary veins (PVs) visible by 2D imaging.
• Establish normal atrioventricular alignments: two separate atrioventricular valves (AVVs) are visualized. Two ventricles of good size are visualized.
• Demonstrate transposed ventriculoarterial connections.
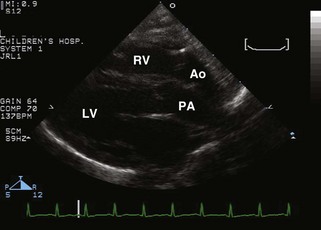
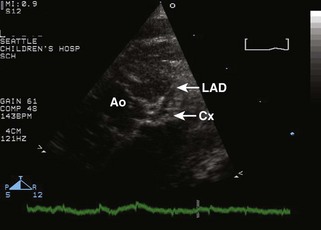
• The more posterior great vessel (GV) arising from the LV bifurcates, identifying it as the PA (Fig. 8-2A).
• The more anterior GV arising from the RV does not bifurcate and is thus the aorta; coronary artery origins may be seen from it.
• Subcostal short axis sweep, base to apex.
• Atrial morphology is again noted to be normal with the systemic veins entering the RA and at least some PVs visualized entering the LA. Assess the size of interatrial communication (patent foramen ovale [PFO] or ASD).
• Ventricular morphology is examined and is consistent with normal ventricular looping. The left-sided ventricle has a smooth septal surface and AVV morphology consistent with an MV (no chordal attachments to the septum). The right-sided ventricle is trabeculated, with chordal attachments of the AVV to the septum. Assess for VSD (size, location).
• Apical views
• Four chambers, of proportionate size, are seen. Confirm that the ventricular morphology appears usual (right-sided ventricle with moderator band, left-sided ventricle with smooth septal surface).
• With anterior angulation, the bifurcating PA is seen arising from the LV. If present, a retropulmonary coronary artery may be seen by angling anteriorly from the MV to the pulmV.
• Parasternal long axis view
• There is mitral-pulmonary fibrous continuity. If not present, consider the diagnosis of double-outlet RV.
• Parasternal short axis view
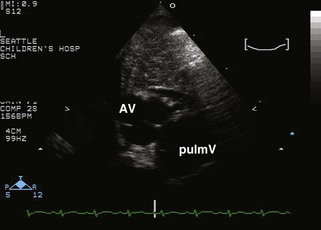
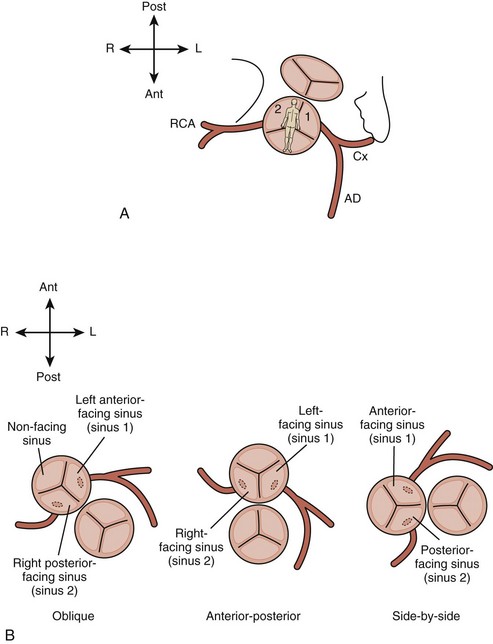
• Note the relative orientation of the semilunar valves (Fig. 8-4). Typically, the AV is anterior and rightward of the pulmV, but can range from directly anterior to rightward and side by side.
• Note morphology of the semilunar valves. The intercoronary commissure of the AV should align with a commissure of the pulmV. If the commissures are not aligned, coronary transfer may be more difficult.
• Note any evidence of VSD. If one VSD is present, be sure to examine the remainder of the muscular septum for additional defects.
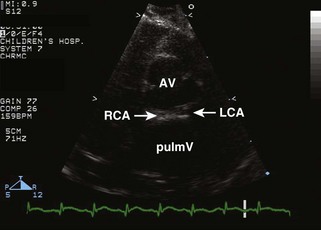
• Evaluate coronary artery anatomy: origins from the sinuses and proximal courses must be clearly visualized.
• Complex coronary artery patterns may substantially increase the risk of the arterial switch operation (ASO). Rarely, a complex coronary artery pattern precludes ASO.
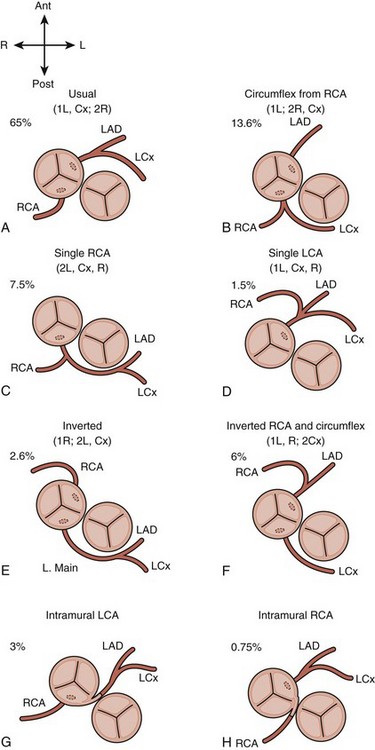
• There are two major conventions for nomenclature, the Leiden convention (Fig. 8-5 and Table 8-1) and the descriptive approach advocated by cardiologists at Children’s Hospital Boston (Fig. 8-6).
• Typically, the coronary arteries arise from the two sinuses of the AV that are adjacent to or “face” the sinuses of the pulmV (see Fig. 8-4).
• In the most common pattern (1L, Cx; 2R) or “usual,” the left coronary artery (LCA) arises from the leftward facing sinus and gives rise to the anterior descending artery and circumflex artery (Cx) (Fig. 8-7). The right coronary artery (RCA) arises from the rightward facing sinus.
• In the second most common pattern (1L; 2R, Cx) or “circumflex from RCA,” the leftward facing sinus gives rise solely to the anterior descending, whereas the RCA, arising from rightward facing sinus, also gives rise to the Cx. The circumflex passes posterior to the pulmonary root to reach the left atrioventricular groove; this segment can often be visualized from the subcostal or apical four-chamber (4C) views (Fig. 8-8).
• Visualization of the coronary arteries is facilitated by decreasing the dynamic range and careful adjustment of focal zone and zoom features.
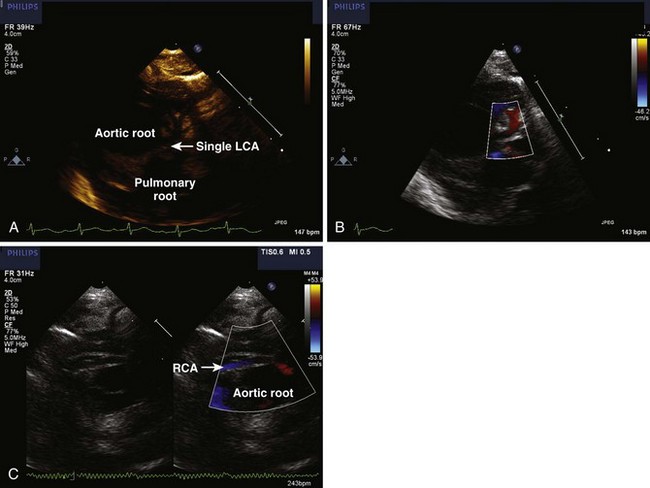
• Be alert for any evidence of intramural coronary artery (passing between the two GVs), which is rare, but can significantly complicate an ASO (Fig. 8-9). A “double border” appearance of the posterior aortic root is noted when a coronary artery passes between the aortic and pulmonary roots.
• Suprasternal notch views
• The usual assessment of arch sidedness and branching is performed. A right aortic arch is uncommon in d-TGA.
• Coarctation is rare unless evidence of right ventricular outflow tract (RVOT) (subaortic) obstruction (RVOTO) is present.
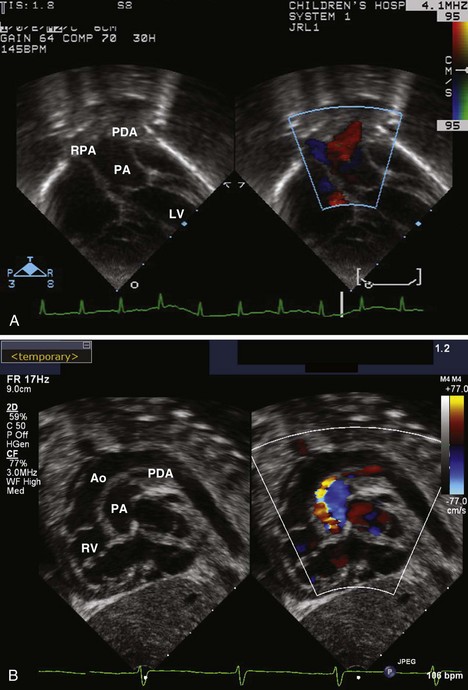
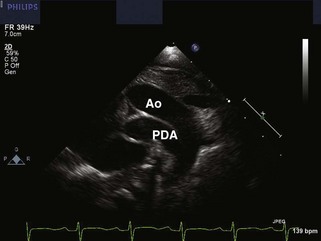
• A PDA is best seen in d-TGA in the sagittal view of the arch due to the parallel orientation of the GVs (Fig. 8-10). The PDA should be assessed for size, direction of shunting, and gradient.
• Transesophageal echocardiography (TEE)
• Given typically excellent transthoracic windows in neonates, TEE is essentially never used for initial diagnosis of d-TGA. TTE is superior for visualization of the GVs and PDA, which are key in this diagnosis.
• Depending on institutional practice, a preoperative TEE may be performed in the operating room. See Chapter 4, Intraoperative Transesophageal Echocardiography, for discussion.
TABLE 8-1 ABBREVIATION CONVENTION USED IN THE LEIDEN CONVENTION FOR DESCRIBING CORONARY ARTERY ANATOMY
| Symbols used are as follows: |
| 1 = sinus 1 |
| 2 = sinus 2 |
| R = right coronary artery |
| L = left anterior descending coronary artery; also designated by some authors AD, as it may not arise from the left. |
| Cx = circumflex coronary artery |
| Comma = major branches originate from a common vessel |
| Semicolon = major branches originate separately |
| Supplemental terms may be used to describe epicardial course and unusual origins. |
| The series of assigned symbols for any given anatomy are enclosed in parentheses. For example: “normal” or “usual” coronary arteries for d-TGA are designated (1L, Cx; 2R), meaning the left anterior descending and circumflex originate from a common vessel from sinus 1, whereas the right coronary artery arises separately from sinus 2. |
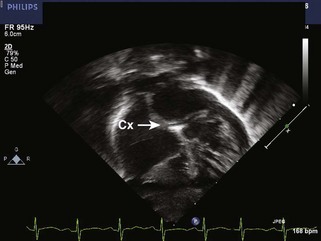
Figure 8-8 As demonstrated in Figure 8-6, when the Cx arises from the RCA (1L; 2R, Cx, or Cx from right coronary artery [RCA]), it reaches the left atrioventricular groove by a retropulmonary course. This may be difficult to see from the parasternal short axis view, but is usually readily evident from the subcostal frontal (shown here) or apical view.
Analysis
• The PDA should be measured at its narrowest point, in the suprasternal notch sagittal view of the arch.
Pitfalls
• The most common pitfall is identifying an RCA and LCA from the two facing sinuses and assuming that the coronary pattern is (1L, Cx; 2R) or “usual,” when in fact the Cx has not been imaged. The second most common pattern, (1L; 2R, Cx) or “circumflex from RCA,” has a retropulmonary Cx, which can generally be well imaged from the subcostal long axis or the apical view. A slow, careful sweep in one of these views from the plane of the MV to the plane of the pulmV should identify this structure. However, the clinical consequence of this mistake is generally not important because both of the two most common patterns are readily amenable to ASO.
• Failure to identify a more complex coronary artery pattern, in particular, one involving an intramural coronary artery, will result in inaccurate counseling as to the risk of the operation. Many surgeons now consider all coronary patterns to be “switchable,” but the risk of the operation is certainly higher with a complex coronary artery pattern. Some surgeons will alter their operative approach due to an extremely complex coronary artery pattern.
• Avoid these pitfalls by careful 2D imaging with optimization of focal zone, zoom, and dynamic range settings with confirmation of structures believed to be coronary arteries with color flow imaging. Follow-up imaging may be needed if the pattern is not determined on the first study. Inspect the area between the two semilunar valves carefully for evidence of a “double border” of the posterior aortic root, which actually represents the coronary passing between the aortic and pulmonary roots. Keep an open mind as to what the coronary artery pattern might be rather than assuming it to be “usual.”
• Multiple muscular VSDs, which occur uncommonly with d-TGA, can be easy to miss in the presence of equal ventricular pressures due to either a large primary VSD or a large PDA. Without a pressure gradient across the ventricular septum (VS), color Doppler across such a defect will not produce an aliased jet. Careful inspection of 2D sweeps of the muscular septum from the subcostal short axis, apical, and parasternal short axis views can identify muscular defects, which can then be confirmed by color Doppler imaging.
Physiologic Data
Acquisition
• Subcostal views
• Perform frontal or short axis color Doppler sweeps of the atrial septum to identify shunts. The mean gradient across the atrial septum is determined by spectral Doppler.
• Short axis color Doppler sweeps of the VS are used to identify any VSD present and determine direction of shunting. Carefully assess any area that the 2D imaging suggests may have a defect (see Pitfalls). Shunting will not be aliased if ventricular pressures are equal due to a large VSD or PDA.
• Acquire peak velocity/peak instantaneous pressure gradient across any VSD in the view that most aligns the Doppler angle with the jet.
• Parasternal short axis view
• Color sweep of the VS is used to identify any VSD present and determine the direction of shunting. Carefully assess any area that the 2D imaging suggests may have a defect (see Pitfalls). Shunting will not be aliased if ventricular pressures are equal due to a large VSD or PDA.
• Acquire peak velocity/peak instantaneous pressure gradient across any VSD in the view that most aligns the Doppler angle with the jet.
• Suprasternal notch view
• Use color Doppler and PW Doppler to determine direction of shunting in the PDA. Use CW Doppler to determine peak velocity and peak instantaneous pressure gradient (PIPG) across the PDA.
Alternate Approaches
Key Points
• Establish the diagnosis of d-TGA by demonstrating the segmental anatomy: normal atrial situs, normal ventricular looping, and d-TGA.
• Mixing between the systemic and pulmonary circuits is required to sustain life. This can occur via an ASD, VSD, or PDA, but is most effective at the atrial level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree