One-year mortality following transplant has been associated with advanced donor age and recipient risk factors including extremes of age, prolonged allograft ischemic times (>200 min), significant renal failure requiring dialysis, and requirement for mechanical circulatory support [4]. For patients who survive beyond the first transplant year, mortality is predominantly related to immunosuppression. Risk factors for 5-year mortality include acute rejection, use of induction therapy (IL-2R antagonists), absence of cell-cycle inhibitors, calcineurin inhibitors, or mTOR inhibitors from the immunosuppression regimen, infection prior to transplant discharge, and dialysis requirements prior to transplant [4].
Primary causes of death within the first year of transplant include graft failure, infection, and acute rejection. Over time, mortality is predominantly driven by malignancy, graft failure, and the development of coronary allograft vasculopathy [4].
6.3.2 CF-LVAD
The survival benefit with mechanical circulatory support in patients with end-stage heart failure was first demonstrated in the seminal Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial [15]. This landmark study clearly showed a profound survival advantage for those who had been implanted with the pulsatile ventricular assist device. Specifically, 1- and 2-year survival rates on device support were 52 and 23 %, respectively, compared to the mortality rate of 75 and 92 % with optimal medical therapy.
However, pulsatile devices are rarely implanted in the current era having been replaced by the newer generation continuous-flow devices [16]. This was motivated by the results of the HeartMate II BTT and DT trials. Specifically, end-stage heart failure patients managed with CF-LVADs benefitted from a two-year actuarial survival of 58 % versus 24 % in the pulsatile device group in the HeartMate II DT trial [6]. Moreover, the complication profile significantly favored CF-LVADs including risk of device failure, strokes, bleeding, and infection. Similarly, CF-LVADs provided hemodynamic benefits for at least six months in patients awaiting cardiac transplantation [17].
More recently, the ADVANCE trial compared the HeartWare HVAD (Framingham, MA) to a contemporary INTERMACS control group [18]. The study findings were notable for a 90.7 % 6-month survival benefit in the HVAD group resulting in its FDA approval as a BTT CF-LVAD strategy. In addition, the study found that the contemporaneous CF-LVAD group had a similar survival rate of 90.1 % at 6 months. The original HeartMate II BTT trial demonstrated 1-year survival of 68 % [17], which improved to 85 % in the more recent data [19]. Other studies indicated that survival improvements have been at least sustained after wider spread of its use [16]. A post-FDA approval study for DT use of HeartMate II showed 2-year survival of 62 and 68 % for INTERMACS profile 4–7 versus 60 % for INTERMACS profile 1–3 [20]. In the INTERMACS Registry, 2-year survival of DT patients (n = 1,694) was approximately 60 %.
Mortality while on device support may arise from a multitude of causes arising from device-related complications and preexisting patient comorbidities. Specific etiologies leading to death include multi-organ failure, infection, bleeding, neurological events, and progression of underlying heart failure [16].
6.3.3 Cardiac Transplant Versus CF-LVAD
Comparing cardiac transplantation and CF-LVAD as heart replacement therapies is problematic owing to the paucity of head-to-head comparative data and the relative differences in patients eligible for either therapy.
Daneshmand et al. comparing their DT LVAD (pulsatile-flow LVAD) patients (n = 60) with patients who underwent high-risk cardiac transplantation (n = 93, the recipients in their extended criteria-alternate list who received a marginal donor heart) [21]. Thirty-day operative mortality and 1-year survival were 2.5 and 82 % for high-risk cardiac transplantation recipients and 6.7 and 77.5 % for DT LVAD patients (p = NS). Three-year survival was, however, better in high-risk cardiac transplantation patients (73 % vs. 50 % in DT LVAD).
The Columbia experience has also found essentially equivalent 1-year survival between DT LVAD, including both continuous and pulsatile devices, and transplant patients older than 65 years of age (83 % vs. 81 %, respectively) [22]. Other single-center experiences attest to similar one-year survival outcomes in patients with BTT CF-LVAD and cardiac transplant [23].
With a focus on LVAD therapy as a potential cardiac transplant replacement strategy, Kirklin et al. recently summarized a large body data from the INTERMACS Registry [24]. Between 2006 and 2011, 1,160 CF-LVADs were registered as a primary DT indication. CF-LVADs led to 1- and 2-year survival rates of 76 and 67 %, respectively. Further analysis of the DT cohort identified a subset of patients who were able to accomplish a transplant-comparable 2-year survival of 80 %. After the exclusion of those patients requiring biventricular support and notable preoperative risk factors, including cardiogenic shock, previous cancer, body mass index greater than 32 kg/m [2], serum sodium less than 130 mmol/L, blood urea nitrogen greater than 50 mg/dL, and previous cardiac surgery, DT patients within this cohort enjoyed 1- and 2-year survival rates of 88 % and 80 % after CF-LVAD implantation (Fig. 6.2). Overall, approximately 20 % of their DT population experienced a 2-year survival equal to or greater than 80 %. This large registry data on DT patients, who in general have more comorbidities than those eligible for transplant, does seem to justify the concept of offering CF-LVAD to selected patients instead of cardiac transplantation in order to achieve equivalent survival outcomes at least in the midterm. However, it is too premature to be conclusive on the survival comparison between these two advanced therapies.
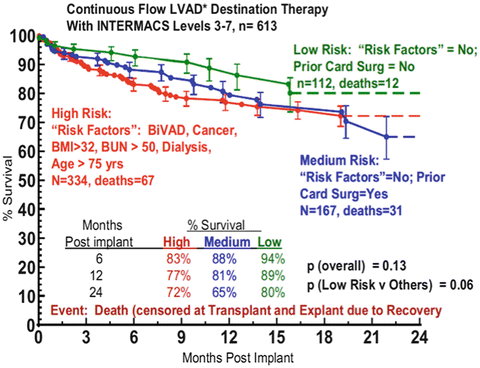

Fig. 6.2
Actuarial survival stratified by high-, medium-, and low-risk patients. “Risk factors” include presence of biventricular support, previous cancer, body mass index (BMI) greater than 32, serum sodium less than 130, or blood urea nitrogen (BUN) greater than 50 [24]. INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device
6.4 Adverse Events
6.4.1 Cardiac Transplantation
Acute rejection, coronary allograft vasculopathy (CAV), renal failure, and malignancy are amongst the most common morbidities that can lead to death in the cardiac transplant patient [4]. These morbidities are often closely linked to the consequences of immunosuppression and the immune interaction between the transplant recipient and the cardiac allograft. Though somewhat less frequent, rejection-related hospitalizations still occur at a rate of 22 % within 1 year and 36 % within 3 years of transplantation [4]. CAV develops in approximately 10 % of recipients within 1 year and more than half by 10 years. The development of CAV is closely linked to both graft failure and ultimate patient death. Survival has improved owing to newer approaches to the treatment of CAV, including the use of statins to lower LDL-cholesterol levels and the addition of mTOR inhibitors to the immunosuppressive regimen. Revascularization with drug-eluting coronary stents has been studied but with variable results. The incidence of renal failure, typically the consequence of calcineurin inhibitor use, is approximately 6 % at 1 year and 16 % at 5 years. Malignancy arises as later complication following transplant with an incidence of non-skin cancer of 1 % at 1 year, 6 % at 5 years, 15 % at 10 years, and 24 % at 15 years. Opportunistic infections may also increase hospital readmissions and the potential risk for death. We have observed an 11 % incidence of pneumonia within the first year after cardiac transplant which was associated with 1-year survival reduction by 9 % [25]. Other non-life-threatening, yet significant post-transplant complications, may further affect a patient’s quality of life. Cardiovascular side effects including hypertension, diabetes mellitus and dyslipidemia, or cosmetic changes resulting from long-standing corticosteroid use have a substantial impact on patients’ quality of life.
6.4.2 CF-LVAD
With the introduction of CF-LVADs, the adverse event profile that significantly complicated the durability of the early generation pulsatile devices has significantly improved. However, the incidence and their clinical significance of such complications remain nontrivial. The reported adverse event rates per 100 patient months are 1.6 for device malfunction, 9.45 for bleeding events, 1.79 in right ventricular failure, 4.66 in cardiac arrhythmia, 8.01 for infection, 1.83 for neurological events, and 1.83 in renal dysfunction in the first year after CF-LVAD implantation [16]. We have reported a 10 % or 0.16 events per patient year incidence of cerebrovascular accidents, including hemorrhagic or ischemic strokes, with the HeartMate II CF-LVAD [26]. Large registry data have demonstrated a nearly 19 % incidence of driveline infection in CF-LVAD recipients at 1 year after CF-LVAD implantation [27].
In addition to these adverse events, longer-term mechanical support has further revealed undesired complications arising from non-pulsatile continuous flow. CF-LVAD use is almost inevitably associated with the development of an acquired von Willebrand syndrome, which, when coupled with obligate device anticoagulation, contributes to high incidence of bleeding events [28]. In addition, nearly 25 % of patients develop de novo aortic insufficiency within the first year following CF-LVAD implantation [29]. In our experience, freedom from moderate or greater aortic insufficiency was 88 and 65 % at 1 and 2 years, respectively. Patients with consequent refractory symptoms or heart failure from device-related aortic insufficiency often require surgical intervention including aortic valve repair or urgent cardiac transplantation [30].
6.4.3 Cardiac Transplantation Versus CF-LVAD
Cardiac transplantation and CF-LVAD therapy have unique and specific complication profiles associated with their use and render head-to-head comparisons challenging. As such, it is difficult to conclude which therapy is “better” from an adverse event profile. Understandably, choice between device and transplant requires a thorough evaluation of underlying patient comorbidity, eligibility for transplant, and ultimately, patient preference.
6.5 Quality of Life
Both cardiac transplant and CF-LVAD therapies result in a remarkable improvement in survival in appropriately selected patients with stage D heart failure. Of equal importance to survival is the restoration and preservation of quality of life and the facility of an active lifestyle after either strategy. Accordingly, nearly 90 % of patients in the first 5 years after transplant have no significant limitations to activity. Approximately 50 % of patients who were transplanted between the ages of 25 and 55 years are employed at 3 years following their transplant [19]. In contrast, quality of life data are somewhat limited in patients on mechanical circulatory support. Available data suggest an important and sustained improvement in general well-being, self-care, and performance of usual activities within the first year following device implantation [16]. However, many patients continue to experience at least some level of emotional distress with their device related to uncertainty, fear of device failure, and anxiety. A review of self-reported patient outcomes found that despite an improvement in overall clinical status beyond the first 3 months after either pulsatile or continuous-flow device implantation, LVAD patients experienced considerably poorer physical and mental health and social functioning both at baseline and 6 months in follow-up as compared to transplant recipients [31].
6.6 Cost
The cost of heart failure management places a huge burden on health-care resources and accounts for 2 % to 5 % of the total health-care budget in most developed countries [32,33]. The expenditure associated with heart transplant and LVAD therapy is in excess of that required in the treatment of various advanced stage medical illnesses [34]. However, the true cost of cardiac transplantation may be underestimated and never be fully realized due to the limited donor heart supply that does not meet the overall heart failure patient population need.
Cost-effectiveness of advanced heart failure management may be further evaluated using economic metrics such quality of adjusted life year (QALY), which takes into account both survival benefit and improvement in quality of life with a medical intervention. It has been suggested that a cost-effectiveness ratio of less than $20,000 per QALY to be very attractive; a ratio of $20,000 to $60,000 per QALY acceptable; a ratio of $60,000 to $100,000 per QALY is less than desirable; and a ratio greater than $100,000 per QALY to be unattractive [35]. As such, the incremental cost-effectiveness ratio of LVADs would need to be at a minimum less than $100,000 per QALY to be considered to be reasonably cost-effective, albeit still more expensive than would be desired. Based on analyses from the HeartMate II Destination Therapy trial, the cost-effectiveness of CF-LVAD therapy was $198,184 per QALY and $167,208 per life year [36]. Even after appreciating that well-conducted cost-effectiveness studies may be limited by many layers of uncertainty in measuring efficacy and economic data, it is clear that LVAD therapy is extremely expensive. Although it is an off-the-shelf treatment modality, its unregulated use may result in an unsustainable economic burden to both patient and society. As such, LVAD therapy, much like the donor heart pool, is also a limited resource, particularly if mechanical support therapy is to be used judiciously in the face of such economic constraints. However, the cost-effectiveness of LVAD therapy will inevitably be dynamic with the evolution of device technology and cumulative clinical experience. As such, modifications to survival and readmission metrics resulting from improved patient selection and overall device management will ultimately impact economic calculations. In fact a significant decrease in cost was demonstrated in patients with HeartMate II CF-LVADs as compared to the early generation pulsatile HeartMate I device after targeted efforts focused on reducing device costs, time in critical care units, and overall hospital lengths of stay resulting from readmissions from complications.
6.7 Functional Recovery of the Native Heart
In some patients, LVAD support facilitates sufficient myocardial recovery of left ventricular function to permit device explantation. In a retrospective analysis of more than one thousand clinical trial patients with HeartMate II LVADs, the overall device explantation rate due to myocardial recovery was 1.8 % [37].
LVAD therapy may also offer the opportunity in combination with other types of strategies to promote significant improvement in the structure and function of the failing myocardium [38]. The Harefield protocol consists of a combined approach of LVAD therapy with aggressive pharmacological neurohormonal blockade (phase I). Phase II of this protocol entails the introduction of clenbuterol, a sympathomimetic amine with β2 agonist properties known to promote physiologic myocardial hypertrophy, to the pharmacological regimen. With this protocol, myocardial recovery was observed in approximately two thirds of patients with a non-ischemic cardiomyopathy [39]. This experience prompted the Harefield Recovery Protocol Study (HARPS), which failed to replicate the result. However, the strategy of combining pharmacological therapy with mechanical unloading to promote myocardial recovery continues to be an appealing therapeutic intervention [40,41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


