Transoesophageal Echo
INDICATIONS FOR TRANSOESOPHAGEAL ECHO
The key difference between TTE and TOE is that for a TOE study the echo probe views the heart from within the patient’s oesophagus rather than via the chest wall (Fig. 7.1). The advantage of this is that it allows for superior image quality – the proximity of the probe to the heart means that the ultrasound does not need to penetrate so deeply, and so higher ultrasound frequencies can be used (giving higher image resolution). The fact that the TOE probe lies behind the back of the heart also means that certain structures – such as the left atrial (LA) appendage and pulmonary veins – can be seen more clearly than with a transthoracic study.
The superior image quality of TOE means that it’s generally indicated in situations where TTE is unable to deliver the image quality required to make a diagnosis. The commonest indications for a TOE study include assessment of:
cardiac source of emboli
atrial fibrillation/flutter, to judge thromboembolic risk (and thus guide decisions on anticoagulation and cardioversion)
suspected or proven infective endocarditis
aortic diseases (e.g. aortic dissection/trauma)
regurgitant heart valves, to judge suitability for surgical repair
prosthetic valves (especially those in the mitral position)
cardiac masses
TOE plays a major role in the cardiothoracic intraoperative setting, particularly in relation to valve repair and replacement, and also in the cardiac catheter laboratory for guiding certain interventional procedures (such as device closure of ASD or PFO). TOE is also useful in the intensive care unit, where the image quality of TTE is often limited in ventilated patients, and as well as being a diagnostic tool, it can also help in haemodynamic monitoring.
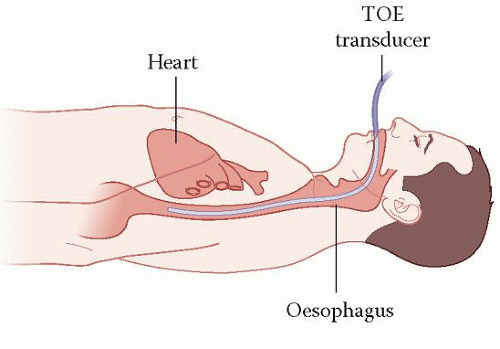 Figure 7.1 Transoesophageal echo (TOE) |
CONTRAINDICATIONS TO A TOE STUDY
Any history of difficulty in swallowing should be investigated before a TOE study can be considered. A TOE study is contraindicated by:
patient refusal
cervical spine instability
any abnormality posing a risk of oesophageal or gastric perforation, e.g. oesophageal obstruction (e.g. stricture, tumour), oesophageal trauma, oesophageal fistula or diverticulum.
Relative contraindications include the presence of clotting disorders, large hiatus hernia (apposition of the probe to the oesophageal wall can be difficult), oesophageal varices or upper gastrointestinal haemorrhage.
PATIENT PREPARATION
The British Society of Echocardiography (BSE) provides very helpful guidance on patient preparation for TOE (see Further Reading). As with any investigation, patients should receive a clear explanation of what a TOE study entails and be offered an information leaflet (ideally at least 24 h prior to the procedure). Inform the patient that a TOE study involves passing a probe into the oesophagus, in a similar manner to having an endoscopy for stomach ulcers, in order to obtain clear ultrasound pictures of the heart.
Inform the patient about the need for local anaesthetic throat spray and discuss with the patient whether or not conscious sedation is to be used (and the consequent need for an escort as appropriate). Conscious sedation is optional – the use of conscious sedation can improve tolerability of the TOE procedure, but does prolong the subsequent recovery period and carries a risk of side effects. Discuss the risks of the procedure. TOE is regarded as a low-risk procedure, but complications can occur and these include:
oropharyngeal trauma (e.g. chipped tooth, pharyngeal laceration)
oesophageal trauma (e.g. laceration, perforation)
laryngeal trauma (e.g. tracheal intubation, laryngospasm)
gastric trauma (e.g. laceration, perforation)
arrhythmias
risks associated with sedation (e.g. respiratory depression).
The overall risk of a major TOE-related complication is reported as being between 0.2 and 0.5 per cent. It has however been suggested that this is an underestimate, as
many of the complications present a day or more after the procedure. The risk of death associated with TOE is estimated to be less than 1 in 10,000.
many of the complications present a day or more after the procedure. The risk of death associated with TOE is estimated to be less than 1 in 10,000.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


