The aim of this study was to compare the outcomes of transcatheter aortic valve implantation using the Edwards SAPIEN valve and the Medtronic CoreValve in patients with annulus of intermediate size (20 to 25 mm). From October 2008 to April 2012, 662 consecutive patients who underwent transcatheter aortic valve implantation were studied at 2 French centers. After propensity score matching, a total of 192 patients with intermediate-sized aortic annulus who had received either Edwards (n = 96, mean age 82.4 ± 7.9 years, 48% men, 61.9% receiving the 26-mm valve) or CoreValve (n = 96, mean age 82.5 ± 7.7 years, 50% men, 64.6% receiving the 29-mm valve) prostheses through the transfemoral approach were studied. Adequate reduction in postprocedural mean pressure gradients was achieved with the Edwards valve and the CoreValve (10.9 ± 4.7 vs 9.1 ± 4.4 mm Hg, respectively, p <0.01). Major vascular complications (5.2% vs 3.1%, p = 0.36), device success (95.8% vs 93.8%, p = 0.52), and 30-day survival (90.6% vs 89.6%, p = 0.81) were similar. The incidence of postprocedural aortic regurgitation grade ≥2/4 and new pacemaker implantation was more frequent in the CoreValve group (14.3% vs 35.5%, p <0.01, and 4.2% vs 18.8%, p <0.01, respectively). There was no significant difference in 1-year cumulative survival rates in the Edwards valve group compared with the CoreValve group (80.1 ± 4.2% vs 75.6 ± 4.9%, log-rank p = 0.31). In conclusion, in patients with annulus of intermediate size, similar device success and short-term and midterm outcomes were achieved with either of the valves, irrespective of the specific complications related to each valve.
Transcatheter aortic valve implantation (TAVI) has emerged as a viable therapeutic option for patients with severe symptomatic aortic stenosis who are ineligible or at high risk for conventional surgical aortic valve replacement. The currently available transcatheter heart valves are the Edwards SAPIEN transcatheter heart valve (Edwards Lifesciences Inc., Irvine, California), a balloon-expandable valve, and the CoreValve ReValving System (Medtronic Inc., Minneapolis, Minnesota), a self-expandable valve. From 2007 to 2011, TAVI was performed using Edwards valves, available in diameters of 23 and 26 mm for patients with annular diameters ranging from 18 to 25 mm, or CoreValve prostheses, available in diameters of 26 and 29 mm for annular diameters of 20 to 27 mm. Consequently, patients with annular diameters of intermediate size (20 to 25 mm) are amenable to treatment with either valve. To date, no direct comparison of clinical outcomes after the implantation of either of the valves has been reported in a homogenous patient population. This was the subject of the study reported here.
Methods
From October 2008 to April 2012, a total of 662 consecutive patients with symptomatic aortic stenosis (valve area ≤1.0 cm 2 ) underwent elective TAVI at 2 French centers (Institut Cardiovasculaire Paris Sud, Massy, France, and Henri Mondor University Hospital, Creteil, France). Patients were selected for TAVI when considered unsuitable or at high risk for surgical aortic valve replacement by consensus between individual centers and heart team discussion. Operative risk was calculated using the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) score and the Society of Thoracic Surgeons predictive risk for mortality score. Patients considered to be at high surgical risk were those with logistic EuroSCOREs >20%, significant co-morbidities, or other risk factors, such as porcelain aorta, not captured by the scoring systems.
Of a total of 662 patients, 170 (14 treated with the Edwards valve and 156 with the CoreValve, 95.2% through the transfemoral approach) were from Henri Mondor University Hospital and 492 patients (376 treated with the Edwards valve and 116 with the CoreValve, 54.8% through the transfemoral approach) were from Institut Cardiovasculaire Paris Sud. Of the initial study population, 230 patients treated through the transapical, trans-subclavian, transcarotid, and transaortic approaches, patients with annular diameters <20 or >25 mm by transesophageal echocardiography, and 19 recipients of 31-mm CoreValve prostheses were excluded from this study. Propensity matching was performed in the remaining 320 patients with annular diameters of 20 to 25 mm who underwent TAVI through the transfemoral approach. Ultimately, a total of 192 patients were enrolled in this study ( Figure 1 ). All patients were already enrolled in the French Aortic National CoreValve and Edwards 2 (FRANCE-2) registry.
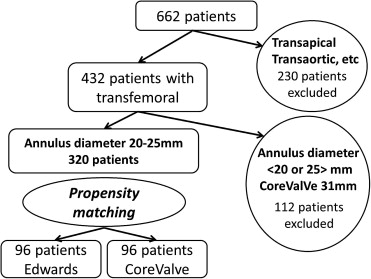
Patient characteristics, echocardiographic data, procedural variables, length of hospital stay, and in-hospital and all-cause mortality rates were prospectively examined. The medical ethics committees at the 2 hospitals approved the protocol of this study, and written informed consent was obtained from all patients before the TAVI procedure.
The type of valve prosthesis was selected by the team of each institution according to its experience and to the diameter of the aortic annulus, which, in the early stages of our experience, was systematically measured using transesophageal echocardiography and more recently by computed tomography using the calculated average annular diameter.
At 1 of the 2 study centers (Institut Cardiovasculaire Paris Sud, Massy, France), the Edwards valve was used preferentially in patients with annular diameters of 20 to 24.5 mm who were amenable to treatment with either of the 2 valves. The CoreValve was implanted in patients whose annular sizes was >24.5 mm and in patients with borderline iliofemoral access precluding the use of 22Fr, or 24Fr Edwards sheaths. Conversely, the CoreValve prosthesis was more frequently used in patients treated at Henri Mondor University Hospital.
All clinical end points of this study were defined according to the Valve Academic Research Consortium criteria. Quantitative variables are expressed as mean ± SD and qualitative variables as numbers and percentages. Chi-square tests or Fisher’s exact tests were used to compare qualitative variables. Comparison of quantitative variables was performed using unpaired Student’s t tests or Mann-Whitney U tests, depending on variable distribution. The propensity score was calculated using a logistic regression model including the following variables; age, gender, body mass index, body surface area, logistic EuroSCORE, estimated glomerular filtration rate, the left ventricular ejection fraction, aortic annular diameter, and New York Heart Association classification. Kaplan-Meier analysis was performed using the log-rank test to compare survival rates between the 2 TAVI bioprostheses. A univariate logistic regression analysis was performed to obtain the odds ratio for 1-year mortality. Thereafter, a Cox logistic regression analysis was performed using variables with p values <0.10 in the univariate analysis, examining their independent associations with 1-year mortality. Statistical significance was defined as p <0.05. Data were analyzed using PASW Statistics version 19.0 (SPSS, Inc., Chicago, Illinois).
Results
Baseline characteristics are listed in Tables 1 and 2 . Significant differences were observed with respect to gender, body surface area, New York Heart Association class, aortic valve area, mean pressure gradient, and the left ventricular ejection fraction ( Table 1 ). After propensity matching, no significant difference was observed with respect to aortic valve area ( Table 2 ). The most commonly used implant was the 26-mm valve in the Edwards group and the 29-mm valve in CoreValve recipients ( Tables 3 and 4 ). Sheath size was significantly larger in the Edwards valve group compared with the CoreValve group. The incidence of acute kidney injury, new pacemaker implantation, and postprocedural aortic regurgitation (AR) grade ≥2/4 was significantly higher in the CoreValve group. The postprocedural mean pressure gradient was significantly higher in the Edwards group. No significant differences were observed with respect to other clinical outcomes. These observations remained unchanged after propensity matching. Postprocedural mitral regurgitation (MR) grade ≥2, logistic EuroSCORE, and transfusion were identified as independent predictors of 1-year mortality by Cox regression analysis ( Table 5 ) after propensity matching. The median follow-up duration of this cohort was 334 days (interquartile range 75 to 1,029) for data analysis carried out before propensity matching and 389 days (interquartile range 126 to 618 days) after propensity matching. The Kaplan-Meier survival curve before propensity matching showed a trend toward an increased 1-year cumulative survival rate in recipients of the Edwards valve compared with the CoreValve (81.8 ± 3.1% vs 76.7 ± 3.7%, log-rank p = 0.06; Figure 2 ). After propensity matching, the Kaplan-Meier survival curve showed that the 1-year cumulative survival rates were 80.1 ± 4.2% and 75.6 ± 4.9% in the Edwards group and the CoreValve group, respectively (log-rank p = 0.31; Figure 3 ).
| Variable | Edwards (n = 170) | CoreValve (n = 150) | p Value |
|---|---|---|---|
| Age (yrs) | 83.0 ± 7.4 | 83.2 ± 7.0 | 0.73 |
| Men | 61 (35.9%) | 83 (55.3%) | <0.01 |
| Body mass index (kg/m 2 ) | 25.8 ± 4.7 | 26.2 ± 4.0 | 0.16 |
| Body surface area (m 2 ) | 1.72 ± 0.18 | 1.78 ± 0.18 | <0.01 |
| Diabetes mellitus | 33 (19.4%) | 33 (22.0%) | 0.57 |
| Hyperlipidemia ∗ | 83 (48.8%) | 74 (49.3%) | 0.93 |
| Hypertension † | 128 (75.3%) | 115 (76.7%) | 0.77 |
| New York Heart Association class III or IV | 148 (87.1%) | 101 (67.3%) | <0.01 |
| Previous myocardial infarction | 21 (12.4%) | 19 (12.7%) | 0.93 |
| Previous percutaneous coronary intervention | 43 (25.3%) | 47 (31.3%) | 0.23 |
| Previous coronary bypass | 21 (12.4%) | 22 (14.7%) | 0.55 |
| Peripheral artery disease | 32 (18.8%) | 35 (23.3%) | 0.32 |
| Cerebrovascular disease | 11 (6.5%) | 13 (8.7%) | 0.46 |
| Chronic obstructive pulmonary disease | 52 (30.6%) | 39 (26.0%) | 0.36 |
| Previous pacemaker implantation | 25 (17.5%) | 28 (20.4%) | 0.53 |
| Logistic EuroSCORE (%) | 21.3 ± 12.2 | 22.9 ± 11.6 | 0.12 |
| Estimated glomerular filtration rate (ml/min) | 55.2 ± 24.9 | 56.1 ± 23.2 | 0.61 |
| Aortic valve area (cm 2 ) | 0.62 ± 0.15 | 0.69 ± 0.18 | <0.01 |
| Mean pressure gradient (mm Hg) | 50.5 ± 17.9 | 45.5 ± 14.8 | 0.04 |
| Left ventricular ejection fraction (%) | 53.1 ± 14.0 | 48.7 ± 15.0 | 0.02 |
| Left ventricular ejection fraction <40% | 39 (22.9%) | 51 (34.0%) | 0.03 |
| Aortic annular diameter measured by transesophageal echocardiography (mm) | 21.7 ± 1.4 | 21.8 ± 1.6 | 0.98 |
| AR grade ≥2 | 32 (20.1%) | 22 (14.8%) | 0.22 |
| MR grade ≥2 | 38 (25.0%) | 21 (15.0%) | 0.03 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


