Transesophageal Echocardiography in Congenital Heart Disease
Jesus Vargas-Barron
Clara Andrea Vazquez-Antona
Angel Romero-Cardenas
Francisco-Javier Roldan
Julio Erdmenger Orellana
Navin C. Nanda
Atrial Septal Defect
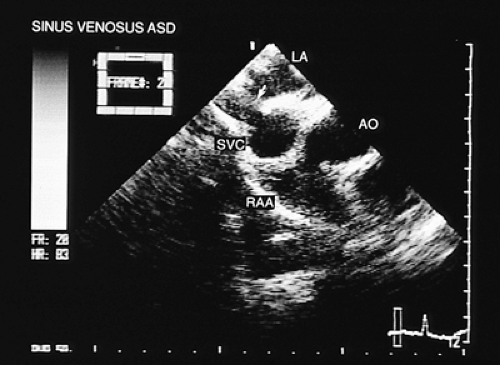
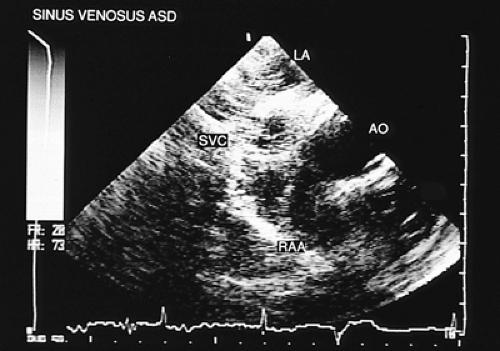
The most common type of atrial septal defect is ostium secundum, located in the middle portion of the interatrial septum. The ostium primum type defect is located in the most inferior portion of the septum, near the crux of the heart. The sinus venosus type of defect can be found in the superior portion of the septum and is often associated with partial anomalous connection of the pulmonary veins.
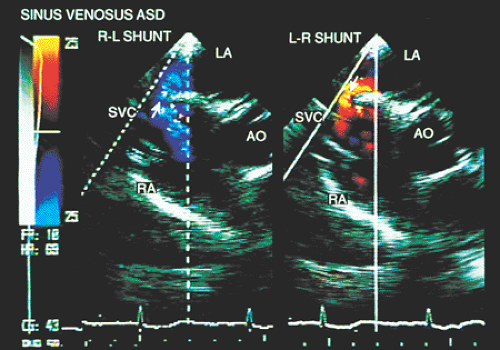
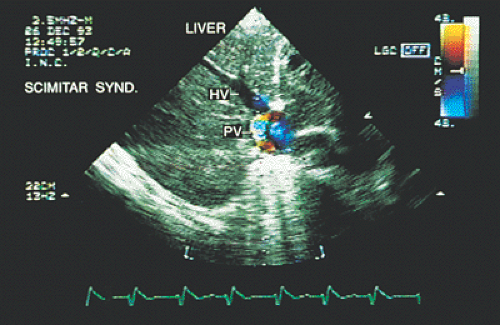
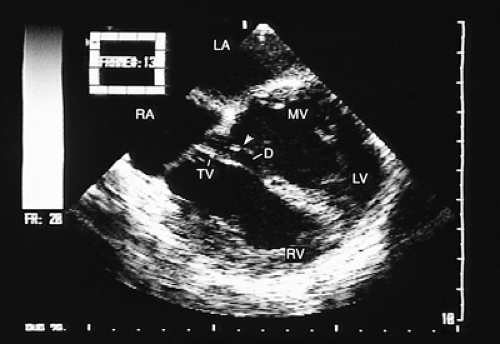
In most patients with atrial septal defect, transthoracic studies provide sufficient information to indicate surgical correction without cardiac catheterization. Indications for a transesophageal echocardiogram, particularly in adult patients, are limited to situations in which conventional transthoracic images do not definitively establish the diagnosis (e.g., obesity, thoracic deformity), when a sinus venosus type defect is suspected (Figs. 8.3.1 and 8.3.2) or when a paradoxic embolism through a patent foramen ovale must be excluded in a patient who has had a stroke (Fig. 8.3.3).
The information obtained from transesophageal two-dimensional recordings should include the following:
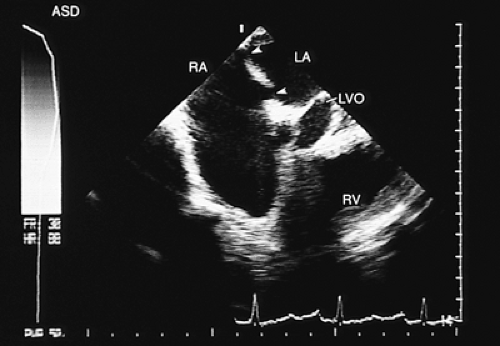
The exact location of the defect or the presence of various defects (Fig. 8.3.4);
The size of the septal defect, with calculation of its area;
Calculation of the shunt flow volume, and the ratio of pulmonary to systemic blood flow;
The site of connection of the four pulmonary veins; and
Any associated defects.
Multiplane images make it possible to determine the exact location of the atrial septal defect. The diameters of the defect can be measured, and the characteristics of the atrial cavity can be examined. Moreover, embryonic
structures such as the eustachian valve can be identified (Figs. 8.3.5, 8.3.6 and 8.3.7).
structures such as the eustachian valve can be identified (Figs. 8.3.5, 8.3.6 and 8.3.7).
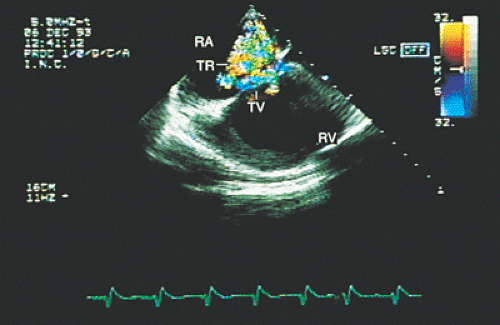
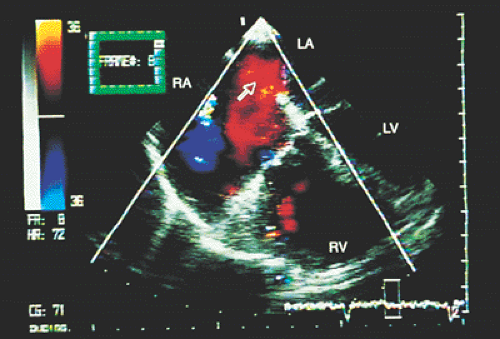
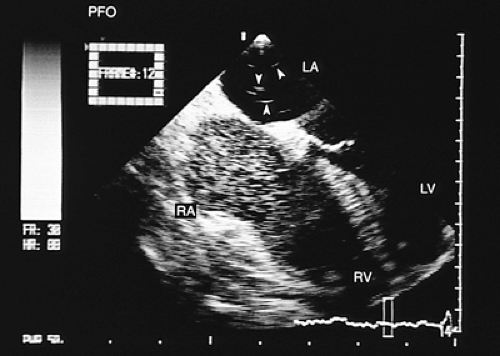 FIGURE 8.3.3. Patent foramen ovale. A contrast study confirms the flow of microbubbles from the right atrium (RA) into the left atrium (LA) (arrows). LV, left ventricle; RV, right ventricle. |
It has been shown that the dimensions of the ostium secundum type defect determined from transverse images correlate well with those obtained in the operating room, as follows:
r = 0.92; P <.001 for horizontal width
r = 0.85; P <.01 for vertical length
The diameter and area of the septal defect can also be calculated from the maximum color flow jet width at the defect site. The defect is assumed to be circular, and comparison with values obtained with those estimated in surgery has shown a fair correlation:
r = 0.73; P = .004
The volume of the shunt is calculated as the product of the area of the defect, mean velocity, flow duration, and heart rate.
A good correlation exists between shunt flow volume calculated by transesophageal echocardiography and that obtained by cardiac catheterization, as follows:
r = 0.91; P <.001
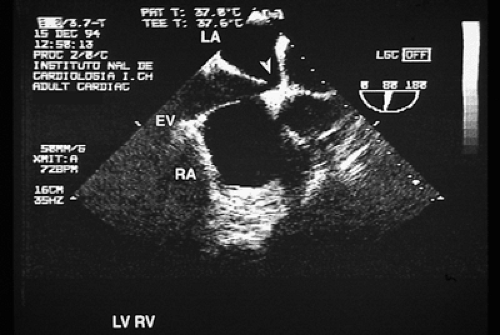 FIGURE 8.3.7. Atrial septal defect. Echocardiogram taken with a multiplanar transducer shows a fossa ovalis type atrial septal defect (arrow). EV, eustachian valve; LA, left atrium; RA, right atrium. |
The comparison of shunt flow volume calculated by transesophageal echocardiography with the pulmonary to systemic blood flow ratio calculated by cardiac catheterization has also shown good correlation:
r = 0.84; P <.001
Once the diagnosis of atrial septal defect is established, the Qp:Qs ratio can be calculated from the flow that crosses the pulmonary valve in systole (Qp) and the aortic or mitral valve (Qs).
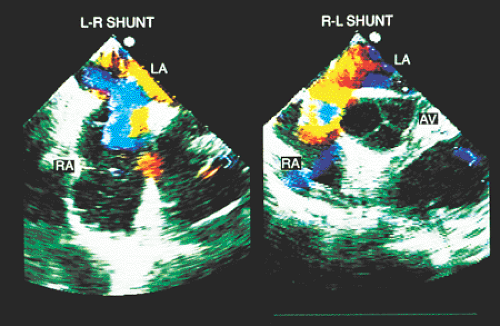
A semiquantitative estimation of mixed shunts is achieved with simultaneous M-mode and color Doppler transesophageal recordings, or by an examination of the positive and negative deflections (spectral analysis) of the flow curve crossing the septum. The planimetry of the positive curve has a certain relationship with the venoarterial shunt, as does the planimetry of the negative curve with the arteriovenous shunt.
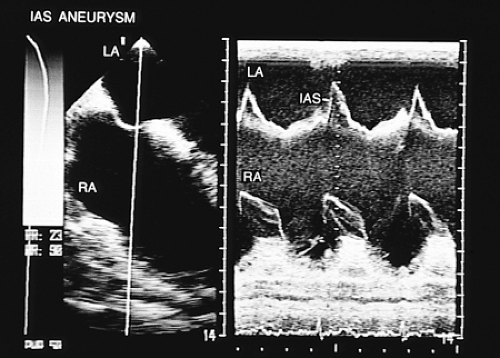
Other pathologies of the interatrial septum exist, such as lipomatous hypertrophy or septal aneurysms, which are identified with relative ease using transesophageal imaging. In lipomatous hypertrophy, the transesophageal images show diffuse thickening of the septum. In patients with septal aneurysms the two signs required for diagnosis are (a) bulging of the fossa ovalis region >15 mm beyond the plane of the atrial septum or >15 mm phasic excursion during the cardiorespiratory cycle and (b) the base of the aneurysm measuring >15 mm in diameter (Figs. 8.3.8 and 8.3.9). Shunting has been detected in up to 83% of aneurysms of the interatrial septum studied by transesophageal imaging using echocardiographic contrast imaging in combination with color flow mapping, and in 41% when only transthoracic examination was performed. Moreover, transesophageal recordings have confirmed that the aneurysms are thrombogenic, which explains their frequent association with paradoxic embolism.
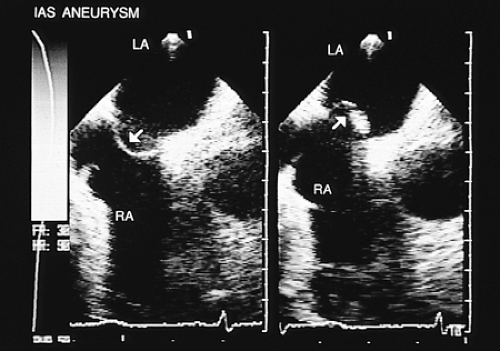 FIGURE 8.3.9. Atrial septal aneurysm. Longitudinal plane views demonstrate both atria. The arrows indicate the aneurysmal movement of the interatrial septum. LA, left atrium; RA, right atrium. |
Transesophageal studies have also served to evaluate the existence of interatrial shunts secondary to percutaneous mitral valvulotomy with balloon catheter, or as a guide in the closure of atrial septal defects with an umbrella-type patch introduced through a catheter.
Partial Anomalous Pulmonary Venous Connection
The most useful noninvasive technique in the diagnosis of anomalous pulmonary venous connections currently available is transesophageal echocardiography. The pattern of pulmonary venous drainage in relation to the atrial septum is best defined with transverse axis imaging (Figs. 8.3.10 and 8.3.11). Transesophageal echocardiography has a sensitivity and specificity close to 100% in the detection of anomalous pulmonary venous connections.
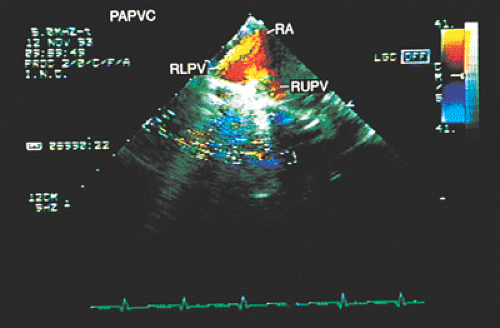
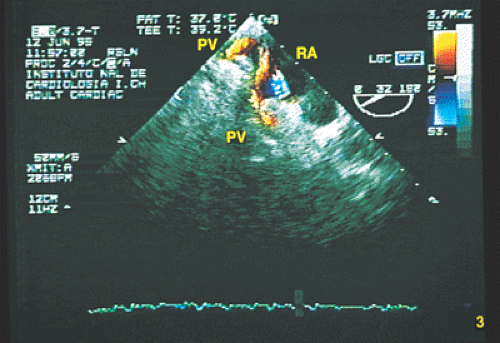
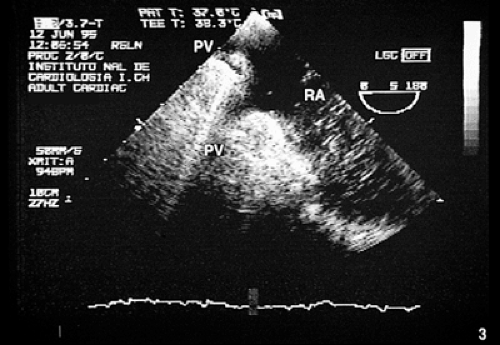
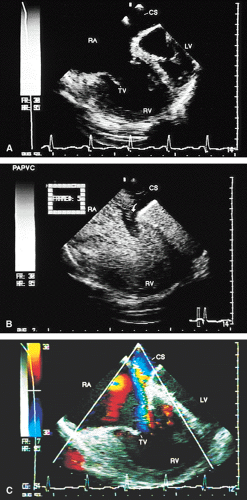
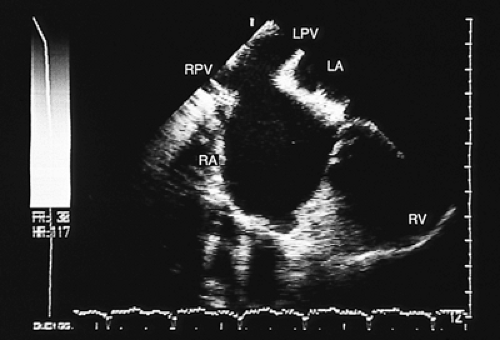
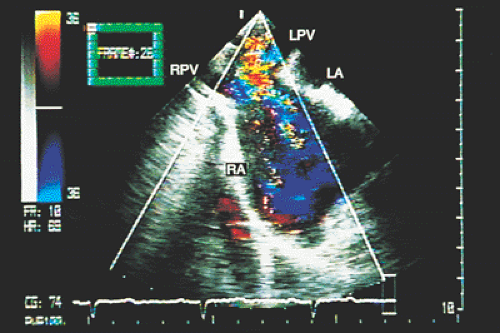
Partial anomalous connections often accompany sinus venosus type atrial septal defects. The most common form is the connection of the right pulmonary veins with the superior vena cava. Less often these veins are found to be connected to the right atrium or the inferior vena cava (Figs. 8.3.12 and 8.3.13); even less common is the connection of the left pulmonary veins with the innominate vein. Direct visualization of anomalous connections, especially of pulmonary veins, has been facilitated by the advent of multiplane transesophageal probes (Fig. 8.3.14). Contrast studies visualize the clearing of microbubbles in the right atrium by anomalous pulmonary venous flow (Fig. 8.3.15).
Total Anomalous Pulmonary Venous Connection
In total anomalous connection of the pulmonary veins, the connection between the four pulmonary veins and the left atrium is absent. The veins drain into the right atrium, either directly or through systems of venous tributaries.
Three types of anomalous connection exist: (a) supracardiac, in which the connection is to the superior vena cava or the innominate vein; (b) cardiac, in which the pulmonary venous return connects to the coronary sinus or directly to the right atrium; and (c) infracardiac, in which the anomalous connection is established with the inferior vena cava or the portal vein. Mixed anomalous connections include at least two of these types.
The sites to which anomalous connections occur are, in decreasing frequency, the innominate vein, by way of a left vertical vein; the coronary sinus; the right superior vena cava; the right atrium; and the inferior vena cava.
In four-chamber transesophageal images, dilatation of the right cavities can be demonstrated and the lack of connection of the pulmonary veins to the left atrium can
be confirmed. The use of transverse plane images makes it possible to recognize the characteristics of the obligatory atrial septal defect (Fig. 8.3.16). In addition, the small size of the left ventricle can be detected, a finding that has been considered a prognostic index in those patients who undergo surgical correction. When the two-dimensional images are complemented with color Doppler and spectral analysis, the magnitude of the venoarterial interatrial shunt becomes evident, and when flow is recorded in the anomalous pulmonary venous channel it is seen to be forward both in systole and diastole. The appearance of a color mosaic with increased flow velocity corresponds to venous obstruction, a situation of considerable clinical significance.
be confirmed. The use of transverse plane images makes it possible to recognize the characteristics of the obligatory atrial septal defect (Fig. 8.3.16). In addition, the small size of the left ventricle can be detected, a finding that has been considered a prognostic index in those patients who undergo surgical correction. When the two-dimensional images are complemented with color Doppler and spectral analysis, the magnitude of the venoarterial interatrial shunt becomes evident, and when flow is recorded in the anomalous pulmonary venous channel it is seen to be forward both in systole and diastole. The appearance of a color mosaic with increased flow velocity corresponds to venous obstruction, a situation of considerable clinical significance.
In anomalous connections of the supracardiac type, transesophageal studies complement the information obtained with suprasternal imaging. In the latter examination, the most common form of supracardiac anomalous connection is well demonstrated: the vertical vein (persistent left superior vena cava) drains into the innominate vein, and from there the venous flow connects with the superior vena cava and finally reaches the right atrium. In contrast, when the anomalous connection is with the right superior vena cava, transesophageal echocardiography shows that the pulmonary veins form a venous confluence posterior to the left atrium that points toward and connects with the posterior portion of the vena cava.
Pulmonary venous anomalous connection can be demonstrated with the greatest facility at the cardiac level in transesophageal images. When the connection is with the coronary sinus, images in the transverse plane show that the pulmonary veins form a retrocardiac confluence, which drains into the coronary sinus at the level of the atrioventricular sulcus. The coronary sinus is very dilated and has an abnormal vertical position (Fig. 8.3.17).
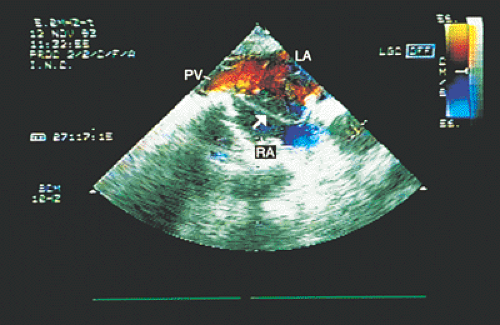
If the anomalous connection is with the right atrium, transesophageal recordings make it possible to establish whether the connection occurs through a large common pulmonary vein or through two to four pulmonary veins that drain separately into the posterior-inferior portion of the atrium (Figs. 8.3.18 and 8.3.19).
In anomalous infracardiac connections, the four pulmonary veins form a common confluence that descends in front of the esophagus, crosses the diaphragm through
the esophageal hiatus, and drains into the inferior vena cava, hepatic or portal vein, or a persistent ductus venosus. Diagnosis of this type of anomaly can be established with subcostal recordings. Low transesophageal and transgastric recordings are very useful, especially when drainage is into the inferior vena cava or hepatic veins.
the esophageal hiatus, and drains into the inferior vena cava, hepatic or portal vein, or a persistent ductus venosus. Diagnosis of this type of anomaly can be established with subcostal recordings. Low transesophageal and transgastric recordings are very useful, especially when drainage is into the inferior vena cava or hepatic veins.
Transesophageal echocardiography, when performed in the cardiac catheterization laboratory, can avoid the excessive use of angiocardiographic contrast medium. In the operating room, it can aid in planning the type of corrective surgery and in the identification of mixed anomalous connections.
Ventricular Septal Defect
The echocardiographic diagnosis of ventricular septal defect in children can be usually established with conventional transthoracic recordings. When technical difficulties exist in obtaining adequate images, especially in adolescents and adults, the transesophageal technique is a good alternative method that, with the advent of biplanar recordings, can confirm the diagnosis and determine the size and location of the defect with precision. The interventricular septum is made up of four principal segments: (a) membranous or perimembranous segment; (b) inlet; (c) outlet; and (d) trabecular segment. Septal defects can exist in one or more of these segments. They produce the features discussed in the following paragraphs in transesophageal echocardiographic studies.
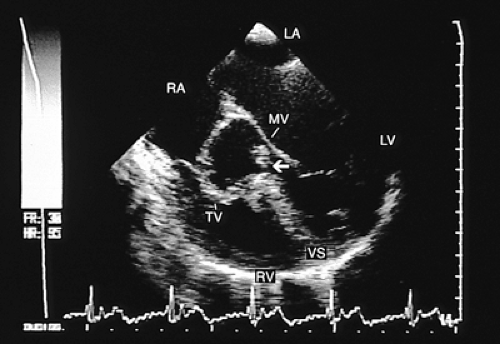
A defect of the membranous septum is the most common. On the right ventricular side it is located beneath and behind the supraventricular crest (infracristal). On the left ventricular side it is located in the subaortic portion, near the commissure of the right and noncoronary leaflets. Diagnosis can be established with transesophageal images in transverse and longitudinal planes (Fig. 8.3.20). In the former, the shunt is seen close to the tricuspid valve and in front of the aorta (at the 8 o’clock position). In longitudinal recordings the defect is located in the basal (superior) third of the septum in a small subaortic portion.
A defect of the posterior or inlet septum may be found behind the tricuspid valve and between the tricuspid and mitral valves. It often forms part of a malformation of the endocardial cushions and is often associated with atrioventricular valve defects. The shunt is located with color Doppler, usually in a four-chamber transesophageal image in the transverse plane.
A defect of the infundibular or outlet septum is another possibility. On the right ventricular side it is located below the pulmonary leaflets; on the left side, it is inferior to the aortic leaflets. Transesophageal recordings in the transverse plane are not useful for recognizing the exact characteristics of the defect. In longitudinal images, however, it is possible to see the ventricular outflow tracts and, with color Doppler, to demonstrate the shunt crossing the defect.
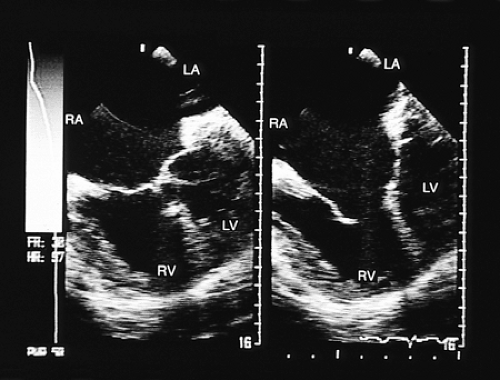
A defect of the trabecular septum is located in an anterior and inferior portion of the septum. Occasionally, multiple trabecular defects are present (Fig. 8.3.21). This type of defect is usually small, although it can also be very large. Such a defect can be difficult to demonstrate in the transverse plane when it is small; identification usually requires biplanar recordings.
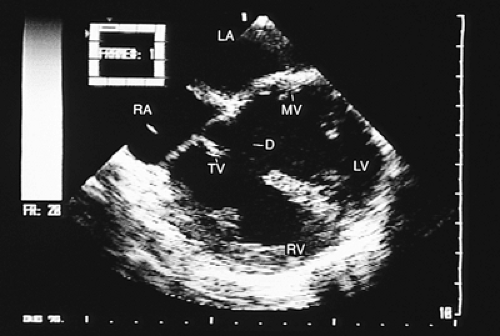
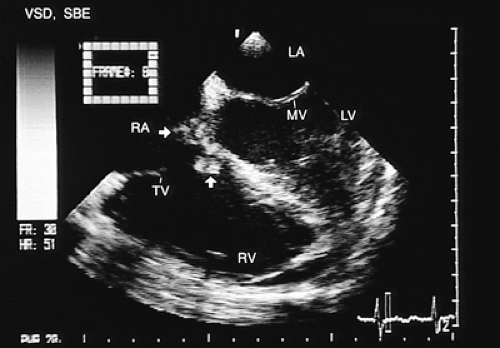
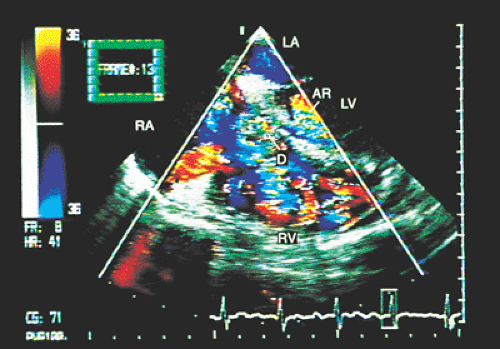
In patients with ventricular septal defects the flow crossing the defect can affect tricuspid structures, and this valvular trauma can promote the development of infective endocarditis. When this occurs, transesophageal recordings with color Doppler aid in identifying the location and magnitude of the ventricular septal defect, the characteristics of the vegetations adhering to the tricuspid leaflets, the existence and severity of the lesions of the leaflets themselves, and the extension of the infectious process to other cardiovascular structures (Figs. 8.3.22, 8.3.23 and 8.3.24).
One of the principal contributions of transesophageal studies in the evaluation of patients with ventricular septal defects is the visualization of the overriding of the atrioventricular valve rings or the straddling of subvalvular structures that cross the interventricular defect and insert in the contralateral ventricle (Fig. 8.3.25).
Transesophageal echocardiography has also been used in the intraoperative evaluation of surgical closure of
ventricular septal defects. It is more useful than epicardial recordings. However, when a patch of synthetic material is used to cover the defect, the patch can produce ultrasonic shadowing in the right ventricle that makes color Doppler evaluation difficult and the demonstration of residual shunts impossible. This interference can be avoided by using “high” (i.e., at the level of the right ventricular outflow tract and the aorta) transesophageal recordings complemented with contrast echocardiography.
ventricular septal defects. It is more useful than epicardial recordings. However, when a patch of synthetic material is used to cover the defect, the patch can produce ultrasonic shadowing in the right ventricle that makes color Doppler evaluation difficult and the demonstration of residual shunts impossible. This interference can be avoided by using “high” (i.e., at the level of the right ventricular outflow tract and the aorta) transesophageal recordings complemented with contrast echocardiography.
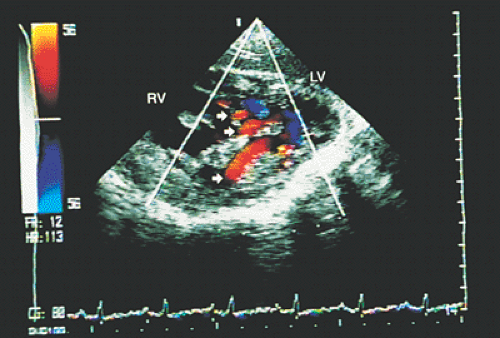 FIGURE 8.3.21. Ventricular septal defect. Transgastric imaging with color Doppler demonstrates multiple ventricular septal defects (arrows). LV, left ventricle; RV, right ventricle. |
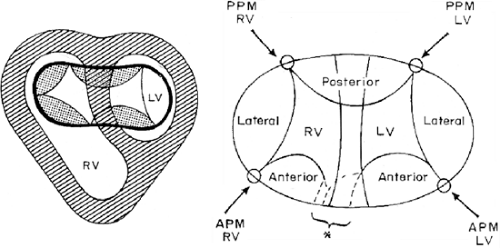
Common Atrioventricular Canal
The common atrioventricular canal is the extreme form of endocardial cushion defects, also called atrioventricular septal defects. In this malformation there is a common AV valve, a defect of the lower portion of the interatrial septum (ostium primum), and a defect of the high, posterior portion of the interventricular septum (inlet).
The AV valve is formed by five leaflets, three of which correspond to the left ventricle—lateral, anterior, and
posterior and two correspond to the right ventricle—one anterior and the other lateral.
posterior and two correspond to the right ventricle—one anterior and the other lateral.
The left anterior and posterior leaflets cross the crest of the interventricular septum toward the right ventricle. These have been called “bridging leaflets” and have variable degrees of tethering to both ventricles (Fig. 8.3.26).
In addition, the characteristics of these bridging leaflets determine whether one or two atrioventricular orifices exist. If the bridging leaflets are separate, there is only one AV orifice, whereas if the leaflets are joined by fibrous tissue, there are two AV orifices.
In infants and children, transthoracic and subcostal echocardiograms are of sufficient quality to clarify the characteristics of the malformation. Transesophageal studies should be limited to those situations in which it is not possible to obtain satisfactory transthoracic recordings, particularly in adults or during the intraoperative evaluation of surgical correction of the defect.
The transesophageal echocardiographic study should demonstrate the morphology of the atrioventricular valve. With transesophageal four-chamber images and transgastric transverse plane images it is possible to determine whether there is a single AV orifice or two orifices (Fig. 8.3.27). When two-dimensional recordings are complemented with color-coded Doppler, existing shunts can be seen. Patients with two AV orifices usually have a single shunt at the atrial level. Patients with a single AV orifice present with both interatrial and interventricular shunts. The Doppler study also aids in determining the existence and degree of regurgitation of the mitral and tricuspid components of the common AV valve (Fig. 8.3.28).
In four-chamber and longitudinal plane images, it is important to identify the atrial and ventricular components of the AV septum, the size of both ventricles, and, particularly, the characteristics of the left anterior bridging leaflet, which defines the most common variants of this malformation according to Rastelli classification. In type A the left anterior bridging leaflet crosses the interventricular septum and inserts in the medial papillary muscle of the right ventricle. Valvular mobility is limited by the insertions in the crest of the interventricular septum. In types B and C, “crossing” of the left anterior leaflet is greater. In type B, after crossing the septum, the leaflet reaches the
medial papillary muscle, which is located in an abnormally apical position. In type C, the leaflet crosses and inserts in the anterior papillary muscle of the right ventricle.
medial papillary muscle, which is located in an abnormally apical position. In type C, the leaflet crosses and inserts in the anterior papillary muscle of the right ventricle.
In patients with separate AV valve orifices, transesophageal study helps demonstrate that the left anterior and left posterior bridging leaflets are joined by a band of fibrous tissue that divides the common orifice.
A “goose-neck” image can be observed in recordings in the longitudinal plane at the level of the left ventricular outlet. This results from the outlet being longer and abnormally far from the interventricular septum because of the more vertical orientation of the AV valve annulus. Obstructions of the left ventricular outlet can be recognized and quantified with the various Doppler techniques.
Transesophageal echocardiography is particularly useful in the operating room because the information it provides allows better planning of the type of corrective surgery, and in the immediate postoperative period it helps identify residual shunts and valvular regurgitation.
Patent Ductus Arteriosus
Patent ductus arteriosus (PDA) involves abnormal communication between the descending aorta and the proximal portion of the left branch of the pulmonary artery. In premature and term infants it can be diagnosed with relative ease using conventional transthoracic recordings. In older children and adults it presents a broad spectrum of clinical manifestations, which vary from the asymptomatic patient with a continuous precordial murmur to a patient with signs of severe pulmonary hypertension.
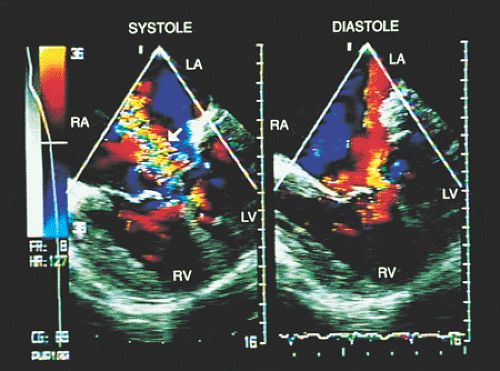
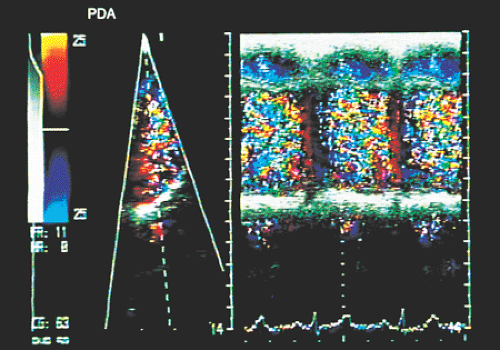
Transesophageal echocardiography is an alternative when satisfactory transthoracic recordings cannot be obtained. It is difficult to visualize the ductus directly in the transverse plane; it may be suspected when there is retrograde systolic and diastolic flow in the pulmonary artery (Figs. 8.3.29 and 8.3.30). The diagnosis is established with biplanar or multiplanar transducers using recordings in the longitudinal plane. In this plane, the descending thoracic aorta should be recorded at the postductal level. The transducer is then withdrawn slowly to obtain sections progressively superior with slight rotation to the right (Fig. 8.3.31). Doppler shows the shunt as a mosaic of colors connecting the aorta to the pulmonary artery (Fig. 8.3.32). A flow with increased velocity in both systole and diastole is recorded on spectral analysis (Fig. 8.3.33).
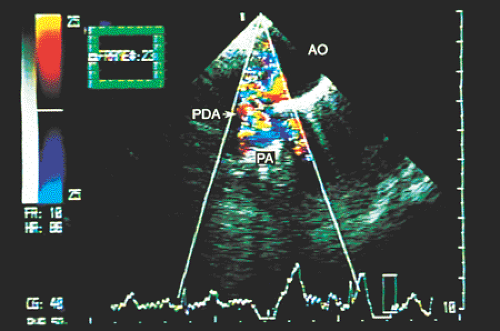 FIGURE 8.3.29. Patent ductus arteriosus. In a high transverse section, color Doppler demonstrates flow through the patent ductus arteriosus (PDA). AO, aorta; PA, pulmonary artery. |
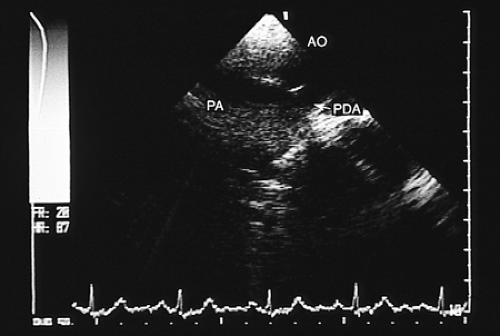 FIGURE 8.3.31. Patent ductus arteriosus. Longitudinal plane imaging at the level of the great arteries directly visualizes the patent ductus arteriosus (PDA). AO, aorta; PA, pulmonary artery. |
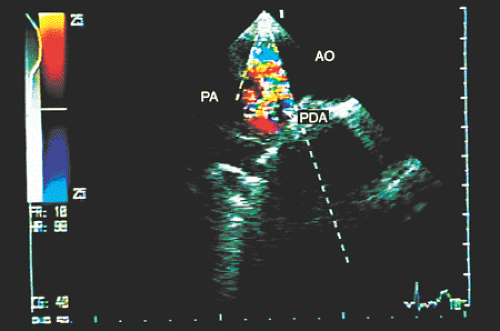 FIGURE 8.3.32. Patent ductus arteriosus. The shunt between the aorta (AO) and the pulmonary artery (PA) is corroborated by color Doppler in longitudinal plane imaging. PDA, patent ductus arteriosus. |
Another application of transesophageal echocardiography in patients with PDA is for the evaluation of the effectiveness of surgical ligation of the ductus. Likewise, in patients with calcification or hypertrophy of the walls of the ductus, residual shunts can exist and can be detected with color Doppler during surgery.
The transesophageal technique has also been used as an aid during interventional procedures. During percutaneous occlusion of PDA with the double umbrella device, transesophageal echocardiography shows the precise position of the device, and, before the device is released, the completeness of occlusion assessed by color Doppler.
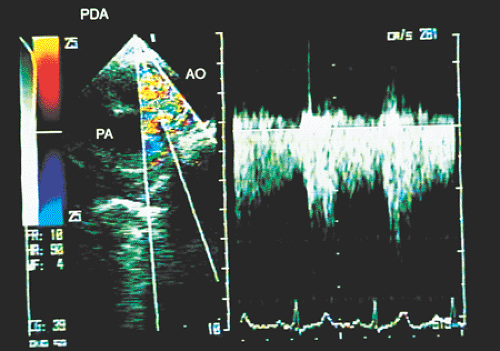 FIGURE 8.3.33. Patent ductus arteriosus.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|