Knowledge and application of the segmental anatomic approach
Knowledge of the spectrum of individual congenital cardiovascular lesions, including common and unusual associated abnormalities
Understanding of the natural history of congenital heart disease, residua, and expected sequelae
Understanding of the dynamic nature of cardiovascular defects
Familiarity with the various surgical and transcatheter interventions available for patient management
Understanding of the effect of adult acquired heart disease on congenital pathology
1.
native anatomy and physiology
2.
how these change over time
3.
types of surgical or transcatheter intervention(s) that may have been performed or for which the patient may be considered a candidate
4.
expected postoperative sequelae and residua
5.
potential postoperative short and long-term changes and complications
6.
influence of adult acquired diseases on underlying congenital malformation or postoperative anatomy and function
A systematic segmental analysis as discussed in Chap. 4 is essential for the echocardiographic evaluation of CHD. This analysis, in most cases performed during childhood, involves determination of atrial and ventricular situs, atrioventricular and ventriculoarterial connections, and the relationship of the great arteries to each other.
The advent and widespread availability of multiplane probes has improved the efficiency and ease of TEE in patients with CHD in general, but particularly those with complex malformations [7]. Imaging the adult with repaired or unrepaired complex CHD is often challenging given the often unusual location and orientation of the heart and extracardiac vessels. The examiner must locate pertinent anatomical structures and align the imaging plane to obtain optimal visualization of anatomy and flow. Needless to say, this requires an in-depth knowledge of the panoply of operated and unoperated congenital malformations prior to performing the study. Review of prior imaging data, including TTE, chest radiography, cardiac-computed tomography, cardiac angiography, and magnetic resonance imaging, is imperative to understanding the underlying anatomy prior to undertaking TEE.
An operator’s innate ability to form a three-dimensional construct of the cardiovascular anatomy in his/her mind and effectively manipulate the TEE probe is essential to acquire the most important images. The full use of two- and three-dimensional echocardiography, color and spectral Doppler, and tissue Doppler facilitates the gathering of important data. It is imperative that “high risk” patients are recognized and managed appropriately when performing TEE (Table 18.2).
Table 18.2
Conditions of increased risk during transesophageal imaging in adult patients
History of cyanotic heart disease with right-to-left shunting |
Concerns related to sedatives: |
Hypoventilation and increased arterial desaturation |
Central nervous system/respiratory depression resulting from faster onset of action of intravenous agents |
Potential acute decreases in systemic vascular resistance leading to hypotension, increased right-to-left shunting, and worsening cyanosis |
Withdrawal of sympathetic tone augmenting myocardial depression |
Hemodynamically unstable patients |
Acute myocardial ischemia or infarction |
Congestive heart failure |
Trauma, if airway, esophagus, neck or oropharynx are involved |
History of radiation to the neck and/or chest |
History of rheumatoid or juvenile rheumatoid arthritis with cervical spinal involvement |
History of scleroderma with esophageal involvement |
Large esophageal varices |
Elderly patients |
Concerns: |
Less tolerance for sedatives |
Cervical spurs may impinge on esophagus |
Esophageal diverticulum |
Potential for arrhythmias |
Coronary artery disease |
Obstructive and/or restrictive lung disease |
Intubated patients |
Concerns: |
Endotracheal tube dislodgment |
Mainstem or endobronchial intubation |
Probe manipulation may impact mechanical ventilation |
Applications of TEE in adults with CHD include the use in the operating room, cardiac catheterization laboratory, electrophysiology laboratory, intensive care units, and other settings [8, 9]. In the operating room, prior to cardiopulmonary bypass, TEE helps refine anatomic diagnosis and may detect previously unsuspected pathology that may require modification of the operative approach (refer to Chap. 15). Once cardiopulmonary bypass is weaned, TEE can be used to evaluate the anatomic, functional, and hemodynamic consequences of the surgical intervention(s). If the initial result is deemed unsatisfactory, return to cardiopulmonary bypass and revision of the repair can be undertaken.
In the cardiac catheterization laboratory, TEE is helpful in guiding transeptal puncture as well as a variety of transcatheter interventional procedures including balloon mitral valvuloplasty or septal defect closure (also refer to Chap. 17) [10, 11]. TEE in adult patients is particularly useful in the identification of thrombus formation and the assessment of findings related to infective endocarditis [12, 13]. This particular patient population may also benefit from transesophageal imaging as this approach overcomes limitations related to suboptimal TTE windows due to prior cardiothoracic interventions or body habitus.
Cardiovascular Malformations in Adults with Congenital Heart Disease and Applications of Transesophageal Imaging
Atrial Septal Defects
Atrial septal defects (ASDs) can go undiagnosed in childhood and in some cases may first come to medical attention during adolescence or adulthood. In such instances, patients commonly present with palpitations and exercise intolerance. The initial echocardiographic examination focuses on diagnosis and hemodynamic assessment of the defect. Precise definition of atrial septal anatomy, identification of coexisting anomalies, quantification of shunt size and right ventricular systolic pressure, are important aspects of the assessment (also refer to Chap. 7). In the adult population, TEE is more sensitive than TTE in detecting atrial communications, especially sinus venosus defects located near the junction of the superior vena cava and right atrium (Fig. 18.1). In general, all ASDs should be imaged in multiple planes in order to determine location and defect size. Determination of adequate rims is important in the case of a secundum ASD for consideration regarding transcatheter device closure (Videos 18.1 and 18.2). Three-dimensional imaging may offer additional benefits in this regard [14]. In general, secundum defects under 40 mm in balloon stretched diameter (Fig. 18.2, Video 18.3) and those with adequate rims are considered potentially suitable for percutaneous device closure [15]. TEE also plays an important role in guiding transcatheter device placement, evaluating residual shunting, and determining device stability (Fig. 18.3, Video 18.4). TEE following transcatheter device closure can identify complications related to the occluder such as thrombus formation [16].
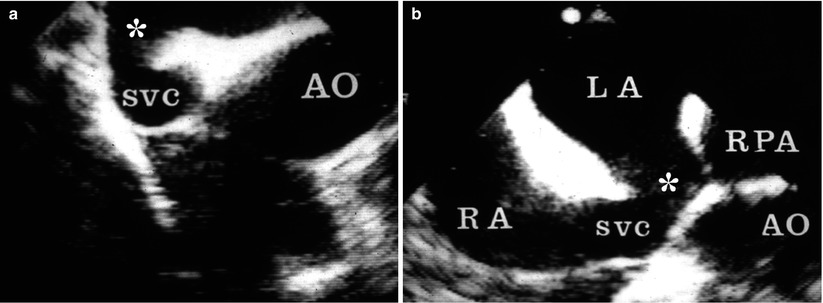
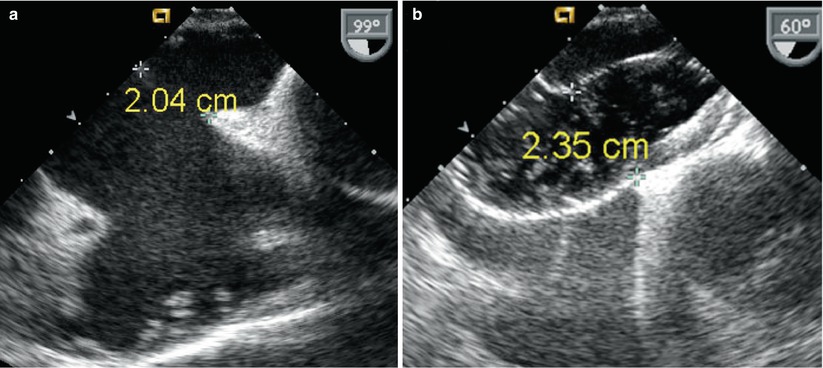
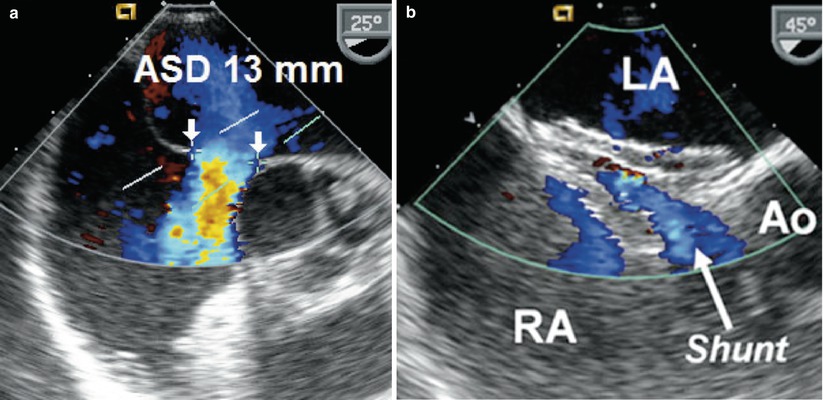

Fig. 18.1
(a) Sinus venosus atrial septal defect imaged in the mid esophageal four chamber view with rightwards transducer rotation. (b) Mid esophageal bicaval view demonstrates the communication between the right atrium (RA) and the left atrium (LA) at the superior vena cava (SVC) and RA junction. AO ascending aorta, RPA right pulmonary artery

Fig. 18.2
(a) Mid esophageal bicaval view of secundum atrial septal defect measuring 2 cm (unstretched diameter). (b) 60° view of the same defect after balloon sizing during transcatheter device placement. The stretched defect diameter measures 2.35 cm in this plane

Fig. 18.3
(a) Secundum atrial septal defect (ASD) measuring 13 mm (unstretched diameter) with thin posterior rim and deficient anterior rim as seen in a mid esophageal view. Color flow Doppler demonstrates left-to-right shunting across the defect. (b) Immediately following successful deployment of an Amplatzer septal occluder device a small amount of left-to-right shunting is noted through the device. Because of the deficient anterior (retro-aortic) rim, the septal occluder is filleted over the posterior portion of the aortic root (Ao). LA left atrium, RA right atrium
Ventricular Septal Defects
Ventricular septal defects (VSDs) may be classified as muscular or non-muscular, aligned or malaligned, and restrictive or non-restrictive. The most common site for an unrepaired VSD in the adult is in the membranous septum, followed by muscular, inlet, and outlet septum.
The ventricular septum cannot be fully imaged in any one or a single plane because it is a curved structure (refer to Chap. 9). Communications in the region of the membranous septum are best seen in the mid esophageal four chamber (ME 4 Ch) view with probe anteflexion. Additional views to define this type of defect include the mid esophageal right ventricular inflow-outflow (ME RV In-Out) and mid esophageal long axis (ME LAX) views (Fig. 18.4, Video 18.5). Continuous wave Doppler allows estimation of shunt velocity and classification into a restrictive versus non-restrictive defect, if the peak velocity across the defect is high or low respectively. Spontaneous closure of a membranous VSD usually occurs by adherence of a redundant aneurysmal tricuspid septal leaflet to the membranous septum. This may be seen in several planes but is best imaged in the 30–60° mid esophageal aortic valve short axis (ME AV SAX) view.
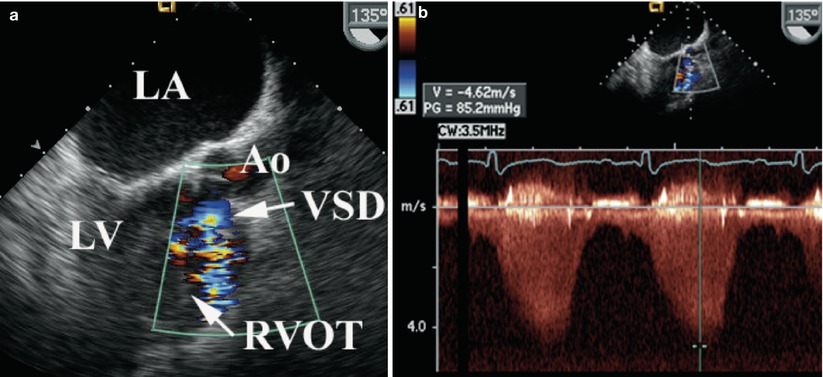

Fig. 18.4
(a) Mid esophageal long axis view in a 135° plane demonstrating shunting across a membranous ventricular septal defect (VSD) into to the right ventricular outflow tract (RVOT) by color flow Doppler. (b) Continuous wave Doppler demonstrates high velocity flow consistent with a restrictive defect. Ao aorta, LA left atrium, LV left ventricle
Imaging and Doppler interrogation of the right ventricle may identify anomalous muscle bundles resulting in a high-pressure proximal chamber and a low-pressure distal chamber characteristic of double-chambered right ventricle (DCRV) (Fig. 18.5, Video 18.6). It has been proposed that this is an acquired lesion potentially related to chronic high velocity VSD flow impacting the right ventricle, however, some controversy still remains regarding this notion. This anomaly is best assessed in the mid esophageal position in the ME RV In-Out view (between 30° and 60°) or from the deep transgastric sagittal (DTG Sagittal) view [17]. Isolated DCRV without a VSD is exceedingly rare.
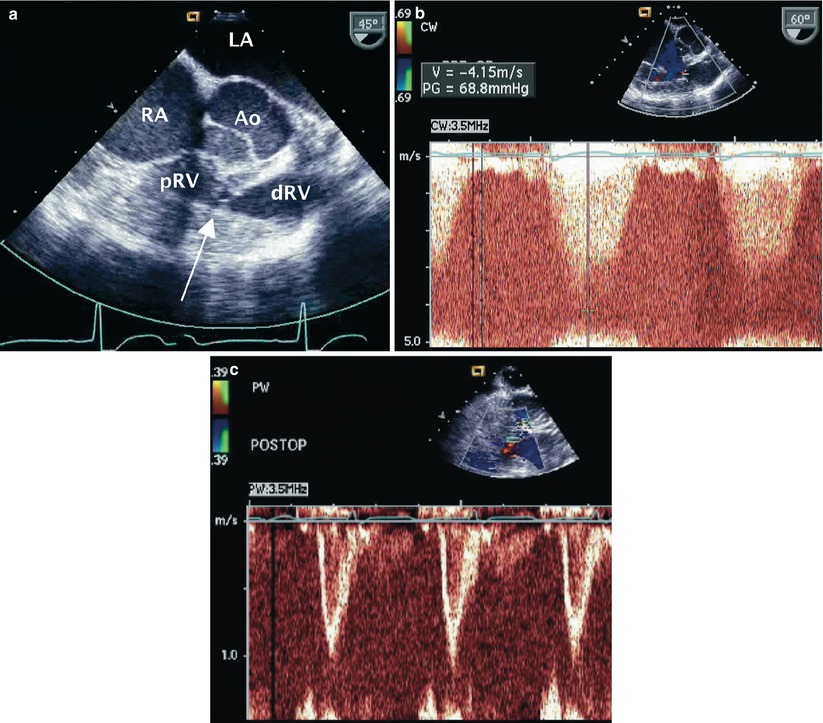

Fig. 18.5
(a) Right ventricular inflow-outflow view at the level of the mid esophagus displaying the typical echocardiographic features in an adult with double-chambered right ventricle. There is mid-cavitary narrowing resulting from a fibromuscular diaphragm (arrow) that partitions the right ventricle into proximal (pRV) and distal (dRV) portions. Note the associated severe right ventricular free wall muscular hypertrophy. (b) Continuous wave Doppler interrogation across the narrowed mid right ventricular region demonstrates a peak velocity of 4.1 m/s consistent with severe obstruction. (c) Following resection of the obstruction pulsed wave Doppler assessment demonstrates low velocity flow across this region confirming the adequacy of the surgical intervention. Ao aorta, LA left atrium, RA right atrium
Occasionally, a patient with a repaired or unrepaired membranous VSD may have a left ventricular-to-right atrial shunt known as a “Gerbode defect”. This is usually a high velocity shunt that may be confused with a jet of high pressure tricuspid regurgitation. This is best seen in the ME 4 Ch and ME RV In-Out views.
A malaligned VSD is associated with great artery annular overriding; typically the aorta overrides the septum as seen in patients with tetralogy of Fallot. The ventriculoarterial connections and malaligned VSD are often best seen in the mid esophageal aortic valve long axis (ME AV LAX) or ME LAX views. Prolapse of the aortic valve (usually the right coronary cusp) into the VSD may cause partial closure of the defect and/or aortic regurgitation. This is seen in outlet (conal or supracristal) defects and is best appreciated in the ME LAX or ME RV In-Out views.
Following surgical or transcatheter VSD closure, interrogation of the septal patch or device for leaks and position as well as assessment of nearby structures is important, preferably done in the operating room or catheterization laboratory at the time of defect closure [18, 19]. In addition, color flow Doppler interrogation of the entire septum in multiple planes may reveal previously undetected small muscular VSDs. TEE plays an important role in transcatheter device occlusion by providing monitoring and guidance during the procedure, decreasing radiation exposure and allowing for detections of complications (refer to Chap. 17).
Atrioventricular Canal Defects
Atrioventricular canal defects, also known as atrioventricular septal defects (AVSDs) or endocardial cushion defects, are a group of malformations that share a common embryologic defect related to the endocardial cushions (refer to Chap. 8). This region influences the development of the atrioventricular septum and the atrioventricular valves.
In the “partial” form, a primum ASD is present with either a restrictive ventricular septal defect (VSD) or intact ventricular septum. This is frequently associated with a commissure or cleft in the anterior mitral leaflet (Fig. 18.6, Video 18.7). The “complete” form of the AVSD is characterized by the presence of a common atrioventricular valve, primum ASD and inlet VSD. The deficiency of the inlet ventricular septum along with abnormalities of the atrioventricular valves (overriding, straddling, and/or cleft) produces an elongated left ventricular outflow tract that has characteristically been described as having a “goose neck” appearance on left ventriculography. Subaortic stenosis is a common association often caused by chordal attachments of the left atrioventricular valve (mitral valve) to the left ventricular outflow septum (Fig. 18.6, Video 18.8).
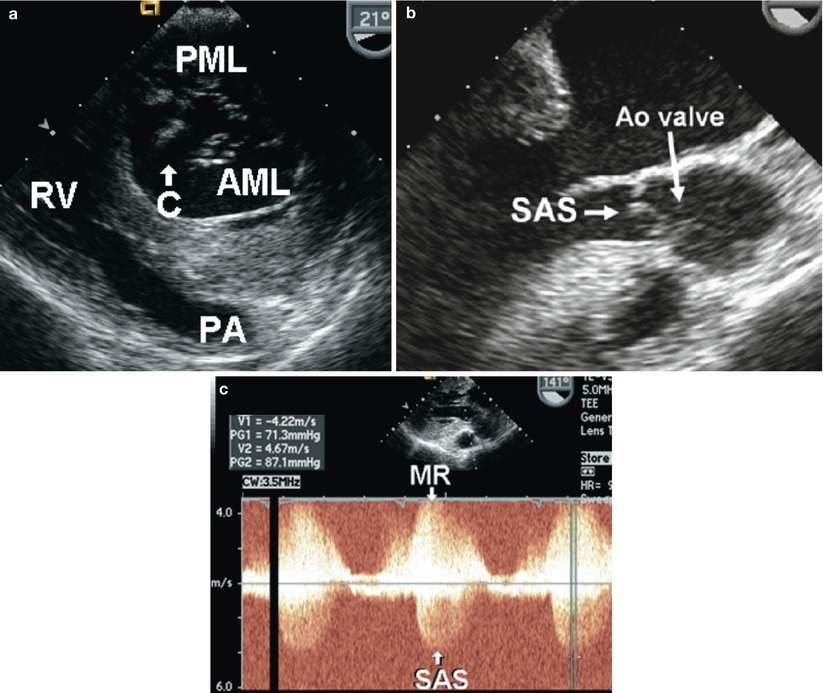

Fig. 18.6
(a) Transgastric basal short axis view of the left ventricle in diastole displays a cleft (C) in the anterior mitral leaflet (AML). The posterior mitral leaflet (PML) is well developed. (b) Mid esophageal aortic valve long axis view demonstrating chordal attachments from the anterior mitral leaflet into the outflow septum causing subaortic stenosis (SAS). (c) Continuous wave Doppler interrogation acquired from a transgastric 141° plane through the region of the mitral leaflet tips and left ventricular outflow tract. Note the jet of mitral regurgitation (MR) directed inferiorly towards the transducer through the cleft in the AML. High velocity systolic antegrade flow away from the transducer is noted, consistent with SAS. Ao aortic, PA pulmonary artery, RV right ventricle
Patients with complete defects frequently undergo definitive surgical repair in infancy. Palliated or unrepaired defects are now rarely seen in adulthood. Palliation is usually in the form of a pulmonary artery band aimed at restricting pulmonary blood flow. Unrepaired defects in adulthood are likely associated with severely elevated pulmonary vascular resistance (Eisenmenger syndrome) and cyanosis related to reversal in the direction of the shunt.
Presentation of an AVSD in the adult patient is most frequently in the form of a partial defect and associated mitral regurgitation. Chordal attachments to the ventricular septum may account for subaortic stenosis and symptomatology. In patients who have undergone a prior repair, TEE is predominantly utilized to evaluate the reconstructed atrioventricular valve(s), with special attention to the degree, etiology, and progression of regurgitation, iatrogenic stenosis, and presence/degree of subaortic stenosis.
TEE evaluation of the mitral apparatus is best done in the mid esophageal and transgastric locations. The various planes that display the mitral valve apparatus are essential for comprehensive visualization of the base and tips of the leaflets with dedicated imaging of the valve scallops from 0° to 180° (refer to Chaps. 4 and 8). The transgastric basal short axis view (TG Basal SAX) that displays the mitral valve in short axis is helpful for direct visualization of the commissure or cleft in the anterior leaflet and all of the scallops of the posterior leaflet (Fig. 18.6, Video 18.7). Color flow Doppler interrogation in this view demonstrates the location and etiology of mitral regurgitation. Short axis views at the level of the mid left ventricular cavity—known as the transgastric mid short axis (TG Mid SAX) views—define papillary muscle anatomy and chordal attachments. In the deep transgastric long axis (DTG LAX) view, flow acceleration in the region of the chordae should arouse the suspicion of subaortic stenosis. The tricuspid valve is also well seen in the ME RV In-Out view and from the transgastric location in the transgastric right ventricular inflow (TG RV In) view.
Interrogation of residual intracardiac shunts, particularly in the case of defects that involve a ventricular level communication or ventriculoatrial shunt, requires the use of multiple planes and is assisted by color flow Doppler imaging.
Anomalous Pulmonary Venous Connections
Pulmonary venous connections are well imaged by TEE (also refer to Chap. 6). In the normal heart, the right and left upper pulmonary veins are usually identified draining into the left atrium at the level of the mid esophagus in the horizontal or transverse plane from zero to 30° with movement of the probe towards the right and left respectively. The left upper pulmonary vein courses into the left atrium posterolateral to the left atrial appendage. The vertical or longitudinal plane at approximately 90° allows visualization of the left and right lower pulmonary veins as they enter the left atrium but modifications of the interrogating plane maybe necessary.
Determination of pulmonary venous anatomy is imperative in patients with ASDs because anomalous pulmonary venous drainage may necessitate surgical intervention as opposed to simple percutaneous closure of an interatrial communication. The most common malformation in this setting is the presence of an anomalous right upper pulmonary vein entering at the junction of the superior vena cava and right atrium in the presence of a superior sinus venosus ASD. Anomalous pulmonary venous connections to the low superior vena cava (between the azygous vein and right atrium) can be visualized by TEE imaging. The probe is positioned at the level of the superior vena cava-right atrial junction with imaging of this area performed in the ME 4 Ch and in the mid esophageal bicaval (ME Bicaval) views. A comprehensive assessment of this defect can be challenging solely on the basis of TEE. In many cases preoperative imaging with modalities such as chest-computed tomography, magnetic resonance imaging, or cardiac catheterization and angiography may be undertaken.
Uncorrected total anomalous pulmonary venous return is an unusual presentation in the adult but may be seen in patients who have received limited or no medical attention, for example, those in third world countries where the resources for appropriate diagnosis and therapies are lacking. A common form of total anomalous pulmonary venous connection seen in adults is the presence of a posteriorly located pulmonary venous confluence chamber into which all four pulmonary veins drain (Fig. 18.7). This chamber is not connected to the left atrium despite the close proximity of these two structures. The confluence typically drains into a greatly dilated coronary sinus that thereafter drains into a dilated right atrium. The presence of a patent foramen ovale or atrial septal defect is necessary to allow venous admixture. In some cases this condition may be confused with cor triatriatum because the close proximity of the venous confluence to the left atrium gives the appearance of a dividing membrane in the left atrium. Less frequently, a vertical vein or other venous structure connects the pulmonary venous confluence to the innominate vein, superior vena cava or travels below the level of the diaphragm to anastomose with the portal vein or a hepatic vein.
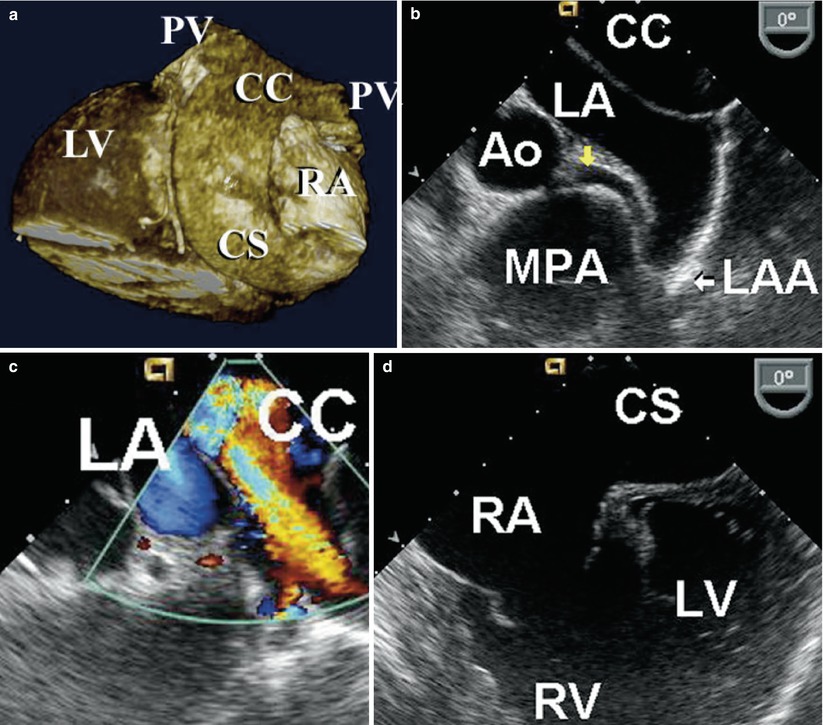

Fig. 18.7
(a) Three-dimensional reconstruction of ECG gated CT angiogram in a 27 year-old cyanotic patient with uncorrected total anomalous pulmonary venous return. Two pulmonary veins (PV) connect to a posterior confluence chamber (CC) that courses inferiorly behind the left atrium (LA) into a dilated coronary sinus (CS) that in turn connects to the right atrium (RA). (b) TEE in the same patient, in the mid esophageal aortic valve short axis view with leftward probe position. Note the posterior CC closely apposing but not connected to the LA. The left main coronary artery (yellow arrow) is seen emerging from the aortic root (Ao) and coursing anterior to the left atrial appendage (LAA) and posterior to a dilated main pulmonary artery (MPA). (c) Color flow Doppler imaging demonstrating low velocity flow towards the transducer from the left sided pulmonary veins to the CC. Note the absence of flow between the CC and LA. (d) Mid esophageal four chamber view with the transducer in a more inferior position at the level of entry of the dilated CS into an enlarged RA. The right ventricle (RV) is also dilated, in comparison to the left ventricle (LV)
Right Ventricular Outflow Tract Lesions
Pulmonary Stenosis
Pulmonary stenosis (PS) may occur at the valvar, supravalvar, or subvalvar level (or any combination of the three levels). Management requires elucidation of the location(s) and severity of the stenosis as well as presence of any associated abnormalities (ASD or VSD). TTE with Doppler interrogation is usually sufficient for the assessment of the adult with PS. However, in patients with supravalvar PS with involvement of the branch pulmonary arteries, TEE provides better visualization of the proximal branch pulmonary arteries, usually best seen at a high esophageal level in the upper esophageal pulmonary artery long axis (UE PA LAX) view. A comprehensive evaluation of the pulmonary vascular tree may not be feasible by TEE, particularly if pathology is suspected distally. In this case alternate diagnostic modalities may be required such as cardiac catheterization, chest tomography or magnetic resonance imaging.
In cases of isolated non-dysplastic pulmonary valve stenosis, transcatheter balloon valvuloplasty is the treatment of choice if the obstruction is considered significant [20, 21]. Transcatheter balloon valvuloplasty provides sufficient relief with an acceptable degree of pulmonary regurgitation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


