Transcranial Doppler Monitoring for Carotid Revascularization
Deepak Sharma
K. H. Kevin Luk
Each year, approximately 795,000 people in the United States experience a new or recurrent stroke, and 87% of these strokes are ischemic.1 The treatment options for atherosclerotic disease of the extracranial carotid artery include carotid revascularization, which may be achieved by open surgical removal of atherosclerotic plaque by carotid endarterectomy (CEA) or endovascular dilation and compression of plaque by carotid artery stenting (CAS). Current guidelines provide a class I recommendation to support CEA and CAS in symptomatic patients with greater than 50% carotid artery stenosis.2 The guidelines also provide a class IIa recommendation for CEA and a class IIb recommendation for CAS in asymptomatic patients with 70% to 99% stenosis.2 In 2010, an estimated 100,000 CEAs were performed in the United States.1 Although the rates of CEA in the Medicare population decreased slightly between 1998 and 2004,3 the rates of CAS increased dramatically from less than 3% of all carotid revascularization procedures in 1998 to 13% in 2008.4
NEUROLOGIC MONITORING DURING CAROTID ENDARTERECTOMY
CEA involves surgical opening of the carotid sheath and exposing the common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) followed by removal of the atherosclerotic plaque. In order to maintain a bloodless surgical field and avoid embolization of air and debris to the cerebral circulation, cross-clamping of the carotid branches both distal and proximal to the CCA bifurcation is necessary. The major perioperative neurologic complications of CEA result from5:
Intraoperative ischemia (due to carotid cross-clamping or due to shunt malfunction if a shunt is used)
Intraoperative and postoperative embolism
Postoperative carotid thrombosis and
Cerebral hyperperfusion
Neuromonitoring during CEA is aimed at early detection of these complications to direct appropriate interventions and minimize neurologic deficits. One option for neuromonitoring is to use regional anesthesia and have an awake, cooperative patient so that cerebral function can be monitored by the patient’s executive function and level of consciousness. A decline in neurologic function serves as a surrogate for cerebral ischemia and indicates the need for shunting or other maneuvers. Unfortunately, regional anesthesia is not a viable option for longer and more complex surgeries and for uncooperative patients. When a general anesthetic is used, it is impossible to obtain real-time feedback from the patient and, therefore, additional monitoring is required to detect cerebral ischemia. Since neither technique (general or regional anesthesia) has been shown to be superior to the other,6 the choice is largely dependent on surgeon preference. Neuromonitoring during CEA may be performed using one or more of the following: electroencephalography (EEG), evoked potentials, cerebral oximetry using near infrared spectroscopy (NIRS), internal carotid stump pressure, and transcranial Doppler (TCD) ultrasonography.7,8,9 Each of these techniques has its own advantages and disadvantages, and there are important differences in their sensitivities and specificities for detecting neurologic complications in the setting of CEA.7,8,9
TRANSCRANIAL DOPPLER MONITORING FOR CAROTID ENDARTERECTOMY
No single monitoring technique has been demonstrated to be completely effective in avoiding neurologic complications associated with CEA. However, TCD is the only monitoring method that is able to detect ischemic, hyperemic, and embolic complications, and it can be used for both intraoperative and postoperative monitoring, as well as for preoperative planning.10 Moreover, it is a continuous, noninvasive, nonradioactive, and nonpharmacologic technique that provides real-time, instantaneous information about changes in cerebral blood flow. Importantly, it is portable and can be performed both in the operating room environment and angiography suite. The report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology provided a Type B recommendation (Class II to III evidence) to support the use of TCD during CEA to detect hemodynamic and embolic events that may result in perioperative stroke.11 The applications of TCD monitoring for CEA can be divided into preoperative, intraoperative, and postoperative (Table 11.1).
TABLE 11.1 APPLICATIONS OF TCD MONITORING FOR CEA | |||
|---|---|---|---|
|
Preoperative Transcranial Doppler
Preoperatively, TCD can be combined with duplex ultrasonography to obtain valuable information for planning CEA. Duplex scanning of the extracranial carotid arteries is discussed in Chapter 8, and the basic principles of TCD are covered in Chapter 9. The TCD assessment prior to CEA includes the following.
Establishing the presence of adequate temporal acoustic windows for insonation of the middle cerebral artery (MCA) during surgery: In 8% to 10% of the general population, bone density does not allow the ultrasound beam to penetrate the skull, leading to failure of insonation with TCD. Inadequate acoustic windows are more common in women and in older subjects,12 and the failure rate has been reported to be higher in some series of CEA, most likely due to the relatively older age of the patient population.8,13
Evaluating the degree of stenosis and collateralization, including the status of the circle of Willis: A complete circle of Willis is present in about 30% to 40% of the general population, and the anterior communicating or posterior communicating arteries may be hypoplastic or nonfunctional. The degree of extracranial carotid stenosis and resulting collateralization can be qualitatively assessed by the “reversal of flow” phenomenon seen on TCD. The ophthalmic artery is one of the first branches from the intracranial ICA and forms collateral connections with the internal maxillary branch of the ECA. Flow in the ophthalmic artery is normally directed toward the eye; a reversal in the flow direction of the ophthalmic artery represents ECA to ICA collateralization.14
Preoperative TCD is a reliable tool for evaluation of the collateral supply through the circle of Willis in patients with ICA occlusion.15 A patent anterior communicating artery is indicated on TCD by a reversal of blood flow in the A1-segment of the anterior cerebral artery (ACA) or by a prompt fall in blood velocity in the middle cerebral artery (MCA) after compression of the nonoccluded contralateral carotid artery.15 Importantly, such carotid compressions must not be performed without first excluding plaques in the carotid system using duplex ultrasonography. In a study evaluating the collateral pathways through the circle of Willis with TCD and cerebral angiography in 40 patients with ICA occlusion, the sensitivity and specificity of TCD in detecting anterior communicating artery collateral supply were 95% and 100%, respectively.15 Collateral supply through the basilar artery is usually indicated by (i) a basilar artery blood velocity of more than 70 cm/s; (ii) a marked increase of basilar artery blood velocity after compression of the nonoccluded carotid artery; and (iii) an evident side-to-side asymmetry of the blood velocity in the posterior cerebral arteries with high velocity ipsilateral to the ICA occlusion.15 For evaluating collateralization via the basilar artery, the sensitivity and specificity of TCD have been reported to be 87% and 95%, respectively.15
Preoperative embolus detection: TCD is also valuable for preoperative detection of cerebral embolization from the carotid lesion and for monitoring therapeutic response using the embolus counts.16
Evaluation of hemodynamic significance of carotid stenosis and vasomotor reactivity: A reversal in the flow direction of the ophthalmic artery represents ECA to ICA collateralization and usually indicates that the lesion in the extracranial carotid artery is long standing and hemodynamically significant.14 A decrease of mean blood flow velocity more than 70% of the basal value during digital common carotid compression and a critical reduction of vasomotor reactivity (no significant increase of mean blood flow velocity in the MCA during the breath-holding test) also indicate a hemodynamically significant carotid stenosis.10
Detecting other intracranial vascular abnormalities: Preoperative TCD evaluation can also be useful for demonstrating stenosis of the MCA, carotid siphon, and other intracranial arteries.10
Although preoperative TCD may provide potentially useful information, some have questioned its utility, arguing that it is not a reliable enough method to modify operative strategy during carotid surgery.13 However, it is generally agreed that, coupled with arteriography, TCD is a good way to study cerebral hemodynamics.13
Intraoperative Transcranial Doppler
Intraoperative monitoring with TCD is aimed at detecting the vascular events leading to cerebral ischemia or hyperemia as they occur, so that appropriate interventions can be made to avoid permanent brain damage.
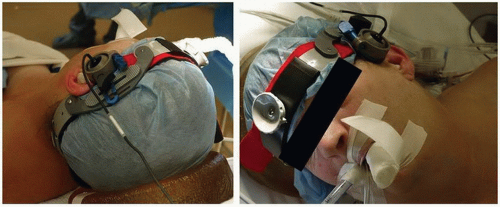
Probe placement and positioning: Typically, a 2-MHz pulsed Doppler ultrasound probe is used to insonate the ipsilateral MCA using standard vessel identification criteria. Some surgeons prefer to monitor the MCAs bilaterally. With either approach, it is important to maintain a constant angle of insonation throughout the operation in order to be sure that any observed velocity changes are related to the surgical procedure, because a change in the angle can affect the flow velocity. Hence, it is crucial that a fixation device be used to stabilize the probe position on the patient’s head and maintain a constant insonation angle during surgery. Figure 11.1 depicts application of the TCD probe with a head frame for a patient undergoing CEA.
Anesthetic considerations: Carotid surgery can be performed under regional or general anesthesia. It is important for the person interpreting TCD findings to be aware of the anesthetic technique being used for CEA because this may affect the cerebral blood flow velocities. In general, compared to the awake state, cerebral
blood flow velocity decreases with induction of anesthesia, indicating decreased cerebral metabolic activity and flow-metabolism coupling.17,18 Thereafter, it remains stable unless the anesthetic depth or concentration of the anesthetic agent is changed. Increasing the concentration of volatile anesthetic agent to greater than 1.0 minimum alveolar concentration may increase the MCA velocity if the blood pressure is constant, indicating cerebral vasodilatation.19 On the other hand, intravenous anesthesia with propofol causes cerebral vasoconstriction and may decrease the MCA velocity.17
While there are no data supporting the relative superiority of one anesthetic agent over the other, it is important for the anesthesia provider to ensure that the anesthetic agent and concentration are not changed during crucial periods of the CEA to avoid interference with interpretation of TCD findings. Another physiologic parameter that may affect the interpretation of intraoperative TCD monitoring is the partial pressure of carbon dioxide.20 Since carbon dioxide is a powerful modulator of cerebrovascular tone, an increase in the partial pressure of carbon dioxide leading to cerebral vasodilatation may cause an increase in the MCA blood flow velocity and vice versa. Therefore, it is important for the anesthesiologist to ensure stable ventilation parameters and avoid inadvertent hypercapnia or hypocapnia during CEA.
Intraoperative monitoring for cerebral ischemia: Carotid cross-clamping during CEA can cause cerebral ischemia, and the primary goal of neuromonitoring is to determine the need for shunt insertion during the period of cross-clamping to carry blood from the CCA to the ICA and maintain cerebral perfusion. While shunting may prevent cerebral hypoperfusion due to cross-clamping, the routine use of an intraluminal shunt may increase the risk of perioperative stroke due to embolic events.21,22 Therefore, selective shunting guided by indicators of cerebral hypoperfusion during test clamping is often advocated.23 It should be acknowledged that there is no definitive evidence that selective shunting is better than routine shunting or nonshunting.24 However, whenever shunting is planned during CEA, TCD is a useful technique to guide the need for shunt insertion as well as to monitor shunt function.
Most surgeons monitor the MCA ipsilateral to the side of CEA. If insonation of the MCA is started with the patient awake, flow velocity monitoring demonstrates an expected decrease after anesthesia is induced. Thereafter, the velocity remains constant or drifts minimally with no decay over time during prolonged anesthesia.25 When TCD is used to determine the need for shunting, the immediate “preclamping” mean flow velocity and the relative change (or more specifically, the percent change) following clamping are used. The magnitude of the MCA mean flow velocity decrease following carotid cross-clamping depends on the status of the collateral vessels, and while this decrease may be small in patients with robust collaterals,21 in others a persistent drop to less than 15% of preclamp values and even complete loss of flow may be seen.21,26 Approximately 10% of patients exhibit an absolute intolerance to unilateral carotid clamping.27 Reported studies have indicated various threshold values for the critical decrease in MCA flow velocity that should trigger shunt insertion,8,9,21,28,29,30,31 and consequently, the TCD criteria for shunting vary among centers.
Electroencephalographic findings consistent with cerebral ischemia typically become evident when cerebral blood flow drops below 10 mL/100 g/min,28,29 which is consistent with an MCA mean flow velocity of 15 cm/s.28 In general, there appears to be good correlation between the decrease in MCA flow velocity and the frequency of EEG changes after carotid clamping.26 In the International Transcranial Doppler Collaborators study, cerebral ischemia was considered severe if the MCA mean flow velocity during the first minute after clamping was 0% to 15% of the preclamp value, mild if 16% to 40%, and absent if greater than 40%.21 These ranges were chosen early in this study and were influenced by ongoing correlative recordings of regional cerebral blood flow (rCBF), EEG, and carotid stump pressure made at some of the 11 participating centers, along with the perioperative rate of severe stroke attributable to intraoperative ischemia.21 Using the above TCD criteria for cerebral ischemia, it was observed that in patients with persisting ischemia (residual flow velocity 0% to 15%), the rate of severe stroke was very high and shunting was protective against stroke.21 At the same time, in patients with no ischemia (residual flow velocity >40%),
the stroke rate was higher with shunting, although not as high as in the unshunted cases with severe ischemia.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree