John G. Webb, John D. Carroll
Transcatheter Therapies for Structural Heart Disease in Adults
Arguably, transcatheter management of structural heart disease dates back more than 30 years with the first successful percutaneous closure of atrial septal defects (ASDs) and the advent of balloon valvuloplasty. However, in the last decade the broad field of structural heart intervention has undergone its most rapid development with improvements in image guidance, the availability of specialized skills, and the completion of pivotal clinical trials of novel devices. This chapter reviews transcatheter interventional therapy for structural heart disease in adults. Although the focus is primarily on acquired conditions, some conditions that are not acquired typically become apparent in adulthood and are also briefly reviewed.
Occlusion of Cardiac Defects
Patent Foramen Ovale
A probe-patent foramen ovale (PFO) is present in 20% to 25% of the adult population. In utero the foramen ovale allows blood to flow across the atrial septum, thereby circumventing the pulmonary circulation (see Chapter 62). This communication normally closes shortly after birth as pulmonary blood flow increases and the flaplike septum primum is forced against the septum secundum. Functional closure is usually followed by permanent fusion of the two flaps. Traditionally considered an innocent remnant of the fetal circulation, a PFO is now assessed in living adults via echocardiographic techniques during the evaluation of those in whom right-to-left shunting may be a central pathophysiologic feature of such diverse conditions as cryptogenic stroke (paradoxical embolism), migraine (shunting of vasoactive substances or microemboli), decompression illness (nitrogen air embolism), and various states characterized by systemic hypoxemia such as platypnea-orthodeoxia (shunting of deoxygenated blood while recumbent).
Paradoxical embolism through a PFO with an arterial embolic event has been the major area of relevance to interventions for structural heart disease with the development of PFO closure devices and delivery systems. The largest target population studied has been patients with cryptogenic stroke, in whom the frequency of finding a PFO is often double that in the general population and in whom the optimal secondary prevention strategy is not well defined. Evidence supporting paradoxical embolism causing a stoke is primarily indirect and presumptive because it is rare to visualize an embolism traversing the PFO. This produces the challenge of separating those with an incidental PFO and an unrelated cryptogenic stroke from those with an enabling PFO and the resultant stroke. Moving beyond the suggestive data showing an association of the presence of a PFO with cryptogenic stroke has been more challenging than originally expected, and this holds even more in the case of migraine. PFO device closure trials have been used to not only assess the safety and efficacy of a device-based strategy but also as a test of the underlying hypothesis of cause and effect. Many single-center studies have been published, most have been retrospective, but all have been observational, often with historical controls. The results of these reports have been summarized in meta-analyses supporting the superiority of device closure, frequently with concomitant antiplatelet therapy, versus medical therapy alone.1,2 Randomized trials have been desperately needed but are difficult to perform, and to date only one migraine trial and three stroke trials have been reported without definitive results proving superiority of device therapy with a high level of certainty.3–5 Defining the target patients most likely to benefit from PFO closure continues to be the central challenge in the setting of low recurrent ischemic stroke rates with medical therapy alone.
Atrial Septal Defect
Secundum ASDs are the most common type of ASD (see Chapter 62). Assessment and characterization are straightforward, and the role of transcatheter closure is well established.7 In adults, ASDs may be detected incidentally or during the evaluation of dyspnea, fatigue, palpitations, or paradoxical embolism. Echocardiographic evaluation (see Chapter 14) is directed toward characterization of the impact of the volume overload (right ventricular and right atrial enlargement), assessment of the position and margins of the defect, evaluation for pulmonary hypertension, and detection of associated defects, including anomalous pulmonary venous return.
Complex defects such as ostium primum and sinus venosus ASDs are more complex and generally require surgical closure, as do very large (diameter >36 mm) secundum ASDs and those with inadequate rim tissue (see Fig. 14-90). Defects associated with pulmonary hypertension and advanced pulmonary vascular disease require in-depth and multidisciplinary assessment of the pulmonary hypertension and its potential for reversibility, the safety of ASD closure, and the use of concomitant medical therapy for the pulmonary hypertension.
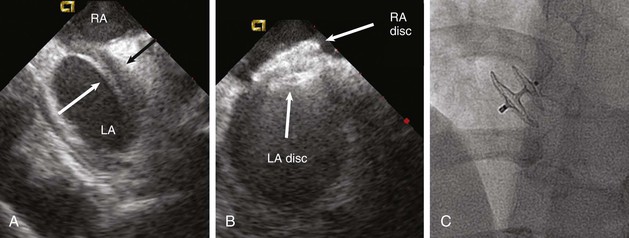
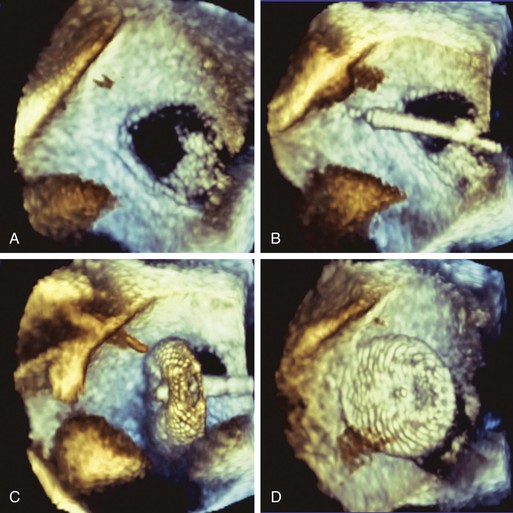
Two devices are widely used for closure of secundum ASDs in North America: the Amplatzer atrial septal occluder (ASO) and the Gore Helex device. ASD occlusion is generally performed via femoral venous access with transesophageal or intracardiac echocardiographic guidance to size the defect, place the device, and evaluate the result (see Figs. 14-87 through 14-91). Complex anatomic subsets may require specialized techniques and equipment, multiple devices, and advanced imaging for planning and guidance, especially three-dimensional transesophageal echocardiography (Fig. 56-2).
ASD closure generally results in a reduction in dilation of the right-sided chambers, as well as improvement in symptoms. However, benefit varies with the degree of chamber enlargement and age. Complications are rare but can include device embolization, particularly when defects are large, complex, or associated with deficient rims and suboptimal device fixation. Atrial fibrillation may occur early as a consequence of atrial manipulation or later as a consequence of chronic atrial dilation and injury. Device thrombosis may occur, but rarely. A particular concern with the Amplatzer ASO device is late erosion involving the roof of the atria or aortic root and subsequently resulting in cardiac perforation and life-threatening cardiac tamponade. The frequency of this serious complication appears to be 0.1% to 0.3%. Risk factors include an oversized implant and a deficient aortic rim. Although erosions generally take place within the first year after implantation, late erosion is rare but may occur.8
Postprocedural care usually includes a transthoracic echocardiogram to assess the final clinical and structural result, 6 months of antiplatelet therapy, and antibiotic prophylaxis for procedures associated with bacteremia.
Ventricular Septal Defect
Acquired ventricular septal defects (VSDs) in adults are relatively rare. They can occur as a consequence of trauma or cardiac surgery. However, the great majority occur in association with acute myocardial infarction. Rupture of the interventricular septum complicates approximately 0.2% of acute infarctions, most often related to a large area of necrotic, weakened transmural myocardium. Patients are usually first seen 2 to 7 days following an acute myocardial infarction—beyond the window of reperfusion strategies—with recurrent chest pain and new-onset heart failure.
Defects that develop in this setting are often irregular, serpiginous, sometimes multiple, and prone to further expansion with time or manipulation. Cardiogenic shock is the norm, and mortality exceeds 90% if left untreated. Even in patients undergoing emergency surgical patch exclusion of a ruptured ventricular septum, mortality ranges from 30% to 60%. Percutaneous closure has been accomplished with various devices, most often with a specialized Amplatzer occluder. An experienced operator can generally implant an occluder safely, but efficacy is variable and mortality remains very high because of myocardial dysfunction and residual leaks. Successful closure is most likely to occur when the defect is small, away from the free ventricular walls and mitral attachments, and chronic. The choice between surgical and percutaneous options must take into account assessment of surgical risk, implications of the defect’s location for surgical exclusion versus percutaneous occlusion, and the need for additional revascularization or valve procedures.
Reoperation for such leaks is accompanied by significant morbidity and mortality, as well as an increased likelihood of recurrent leaks. Medical management may be the best option for many such patients, but percutaneous closure might be an option in carefully selected patients (Fig. 56-4). To date, the role of percutaneous closure has been limited by technical difficulty and modest efficacy. However, results have improved with recent advances in imaging (particularly three-dimensional transesophageal echocardiography), technique (steerable transseptal catheters, apical access), and improved closure devices.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree