54 Transcatheter Therapies for Congenital Heart Disease
 Pulmonary Balloon Valvuloplasty
Pulmonary Balloon Valvuloplasty
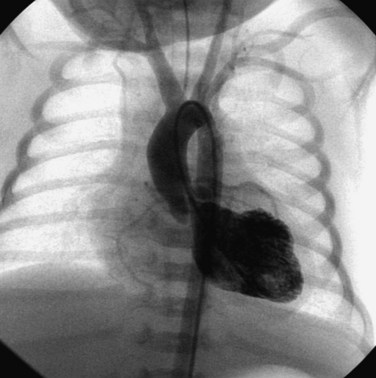
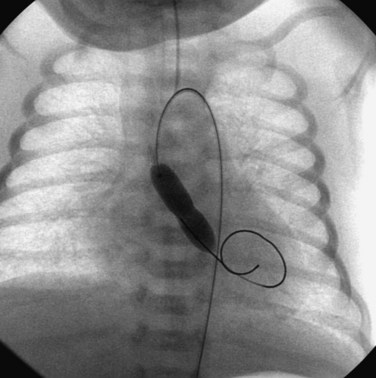
Pulmonary valve stenosis is a common disorder, accounting for approximately 8% of all congenital heart disease.1 Except for neonates with critical pulmonary stenosis, untreated patients often survive well into adulthood.2 However, when more than mild obstruction to right ventricular outflow is present, pulmonary valve stenosis should be relieved to prevent progression of obstruction,3 progressive right ventricular hypertrophy, and right ventricular myocardial fibrosis and dysfunction. If left untreated, significant pulmonary valve stenosis eventually produces clinical symptoms such as fatigue, dyspnea, and exercise intolerance. These long-term sequelae are more likely to be avoided if pulmonary valve stenosis is treated in childhood. Nevertheless, treatment is indicated at any age if hemodynamically significant pulmonary stenosis is documented. Since its introduction in 1982 by Kan et al.,4 percutaneous balloon valvuloplasty has been shown to provide substantial relief of right ventricular outflow tract (RVOT) obstruction in patients with valvar pulmonary stenosis. Balloon pulmonary valvuloplasty can be performed safely and is obviously much less invasive than a surgical procedure. It is therefore regarded as the treatment of choice for patients with moderate to severe isolated pulmonary valve stenosis. In congenital pulmonary valve stenosis, the valve leaflets are thickened and the commissures fused to varying degree. The lines of commissural fusion may appear as two or three raphes extending from the valve annulus to a small central orifice.5 During childhood and young adulthood, the pulmonary valve leaflets are typically supple, doming upward during systole (Fig. 54-1). In older adults, pulmonary valve calcification may occur and may lead to diminished leaflet mobility. A much less common form of pulmonary stenosis has been referred to as “pulmonary valve dysplasia.”5,6 It often occurs as a familial trait or as part of Noonan’s syndrome. A dysplastic pulmonary valve is characterized by thick, cartilaginous valve leaflets with poor mobility (Fig. 54-2). The pulmonary valve annulus is often hypoplastic, and there may be little or no commissural fusion. In isolated pulmonary valve stenosis, balloon dilation reduces the degree of valvar obstruction by separating fused commissures or by tearing the valve leaflets themselves.7,8 Patients with severe pulmonary valve dysplasia with marked hypoplasia of the annulus and absence of commissural fusion may have minimal improvement after balloon valvuloplasty.9 However, because a spectrum of pulmonary valve dysplasia exists, some patients with this disorder may derive substantial benefit from the balloon valvuloplasty procedure.10
Technique
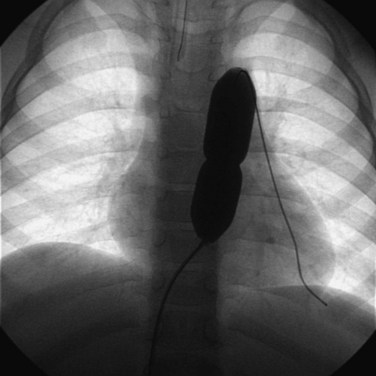
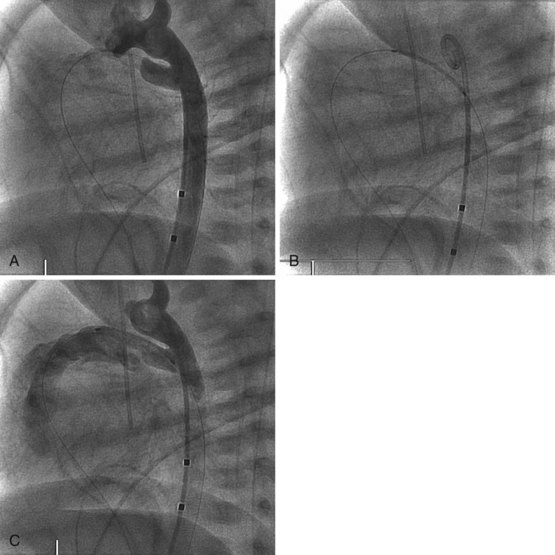
Balloon pulmonary valvuloplasty is technically less challenging than balloon mitral or aortic valvuloplasty procedures. It is performed entirely transvenously and without the need for a transseptal left heart catheterization. The procedure also differs from aortic valvuloplasty in that the use of an oversized balloon, approximately 25% larger than the valve annulus diameter, is required for the most effective relief of obstruction. In general, we believe that pulmonary valvuloplasty is indicated for isolated pulmonary valve stenosis if the resting peak systolic pressure gradient exceeds 40 mm Hg in the presence of a normal cardiac output. In an infant with critical pulmonary stenosis, a right-to-left atrial shunt, and a patent ductus arteriosus, valvuloplasty is indicated even though the measured transvalvar gradient may be less than 40 mm Hg. Balloon pulmonary valvuloplasty is usually performed using a percutaneous transfemoral approach. Right heart catheterization documents the severity of the lesion. Right ventricular angiocardiography is performed to confirm the nature of the lesion and measure the diameter of the pulmonary valve annulus. Typically, the lateral projection is best suited to this purpose. Once the decision is made to proceed with valvuloplasty, an end-hole catheter is advanced to the left pulmonary artery. The left pulmonary artery provides better wire and balloon stability than a right pulmonary artery position. An exchange-length guidewire is advanced to the distal left pulmonary artery and the end-hole catheter is removed. The balloon valvuloplasty catheter is then inserted over the exchange wire. A balloon valvuloplasty catheter is used whose inflated balloon diameter is approximately 15% to 25% larger than the pulmonary valve annulus diameter (Fig. 54-3). Balloon oversizing improves valvuloplasty effectiveness, and injury to the pulmonary valve annulus is unlikely when balloons smaller than 140% of the annulus’s diameter are used.11,12 If the pulmonary valve annulus exceeds 20 mm or if the single balloon catheter required is too large for safe introduction into a patient’s femoral vein (Fig. 54-4), we recommend a double-balloon technique, with two balloons positioned across the valve and inflated simultaneously. The effective dilating diameter of two equal-sized balloons can be calculated based on cross-sectional area or on circumference. The sum of the balloon diameters by the circumference and area methods is 120% and 130% of the equivalent single–balloon diameters, respectively. Thus the operator first selects the optimal single-balloon size, multiplies this diameter by 1.2 or 1.3, and then selects two balloons whose diameters are half of that product. Once inserted, the balloon valvuloplasty catheter is advanced across the valve and positioned with the valve at the midportion of the balloon. Partial balloon inflation, with a mixture of saline and contrast, is helpful to determine the precise location of the valve on the balloon. The valvuloplasty balloon (or balloons) is then inflated by hand until the waist produced by the valve on the balloon disappears. The period of balloon inflation is kept as brief as possible to minimize the obstruction to right ventricular outflow. Typically three or four balloon inflations are performed with minor adjustments in balloon position to ensure adequate dilation of the pulmonary valve. After the dilation is completed, the valvuloplasty catheter is withdrawn and replaced with a diagnostic catheter. The residual RVOT gradient and cardiac output are measured to document the effectiveness of the procedure. A repeat right ventricular angiogram may be performed if necessary to document the degree of subvalvar infundibular narrowing (which may be increased immediately after valvuloplasty) present at this point (Fig. 54-5).
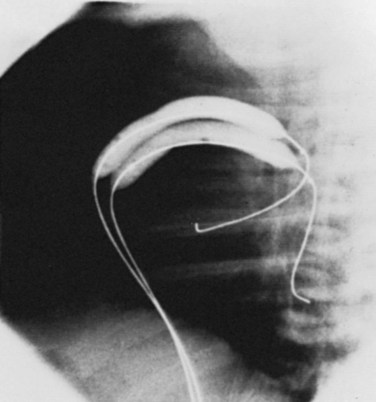
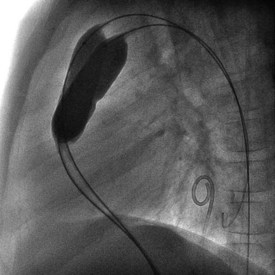
Figure 54-3 Lateral view during pulmonary balloon valvuloplasty in the same infant whose valve is demonstrated in Figure 54-2. The dilation balloon is inflated across the valve, and the impression of the valve annulus is clearly evident near the middle of the balloon.
Acute Results
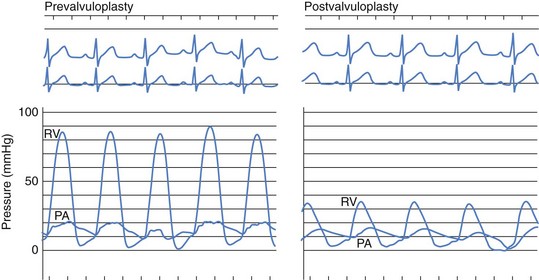
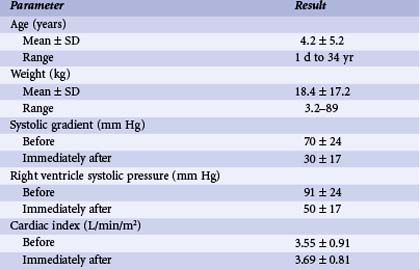
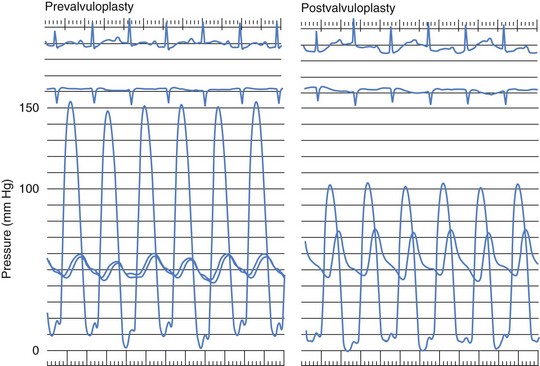
In patients with isolated pulmonary valve stenosis, percutaneous balloon valvuloplasty can be expected to provide excellent relief of RVOT obstruction (Fig. 54-6). Numerous studies have clearly documented significant acute reduction in the peak systolic pulmonary valve gradient to 30 mm Hg or less (i.e., mild residual stenosis). In their landmark article, Kan and colleagues reported the acute effects of valvuloplasty in an 8-year-old child with pulmonary stenosis.4 The procedure decreased the peak transvalvar gradient from 48 to 14 mm Hg and was performed without significant complications. Other studies have subsequently confirmed Kan and associates’ initial observation that valvuloplasty provides impressive gradient relief acutely.11,13–19 The largest published clinical series of balloon pulmonary valvuloplasty was reported by the Pediatric Valvuloplasty Registry.13 This registry reported the acute results of pulmonary valvuloplasty performed in 784 patients between 1981 and 1986. Overall, balloon dilation resulted in an acute decrease in the peak systolic pressure gradient from 71 to 28 mm Hg. The residual pressure gradients immediately after valvuloplasty were ascribed in part to subvalvar infundibular obstruction related to right ventricular hypertrophy. Effectiveness of the procedure was not related to age (the series included 35 adults older than age 21 years), but a larger residual gradient was observed in patients with a dysplastic pulmonary valve. The Pediatric Valvuloplasty Registry described five major complications (0.6%), primarily confined to infancy. There were two procedure-related deaths (0.2%) in 2 infants and 1 neonate in whom RVOT perforation and tamponade occurred. In 2 children, severe tricuspid regurgitation developed related to injury to the tricuspid valve apparatus. Minor complications reported included femoral venous thrombosis, hemorrhage, and transient arrhythmias. The authors’ experience with percutaneous balloon pulmonary valvuloplasty is consistent with that reported from other centers. During our first 10-year experience, from 1982 to 1992, balloon valvuloplasty was performed in 90 patients with isolated pulmonary valve stenosis (Table 54-1). These patients ranged in age from 1 day to 34 years (4.2 ± 5.2 years [mean ± SD]) and in weight from 3.2 to 89 kg (18.4 ± 17.2 kg). Valvuloplasty was performed with balloons ranging in diameter from 5 to 20 mm, and the double-balloon technique was used in 16 instances. Overall, valvuloplasty decreased the peak systolic valve gradient acutely by 57%. The peak pulmonary stenosis gradient decreased from 70 ± 24 to 30 ± 17 mm Hg after valvuloplasty (P < 0.0001). Right ventricular systolic pressure decreased from 91 ± 24 to 50 ± 17 mm Hg (P < 0.0001), and right ventricular end-diastolic pressure decreased from 9.8 ± 3.2 to 8.5 ± 2.5 mm Hg (P < 0.01). There was no significant change in heart rate or cardiac output after the procedure. In our experience, there has been no relationship between patient age or size and the residual pulmonary valve gradient after valvuloplasty.
TABLE 54-1 Pertinent Data before and after Balloon Pulmonary Valvuloplasty in 90 Patients
| Parameter | Result |
|---|---|
| Age (years) | |
| Mean ± SD | 4.2 ± 5.2 |
| Range | 1 d to 34 yr |
| Weight (kg) | |
| Mean ± SD | 18.4 ± 17.2 |
| Range | 3.2–89 |
| Systolic gradient (mm Hg) | |
| Before | 70 ± 24 |
| Immediately after | 30 ± 17 |
| Right ventricle systolic pressure (mm Hg) | |
| Before | 91 ± 24 |
| Immediately after | 50 ± 17 |
| Cardiac index (L/min/m2) | |
| Before | 3.55 ± 0.91 |
| Immediately after | 3.69 ± 0.81 |
Infants
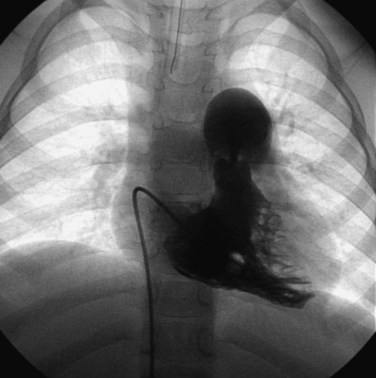
Newborns and infants with critical pulmonary stenosis or atresia are frequently critically ill and hypoxemic (because of a right-to-left atrial shunt) and may have associated hypoplasia of the right ventricle and tricuspid valve (Fig. 54-7). Because of these factors, in addition to the presence of severe RVOT obstruction, it is a technical challenge to successfully catheterize the pulmonary artery and properly position a valvuloplasty balloon across the RVOT in these infants.16,17,19–21 In infants with critical pulmonary stenosis, we prefer to perform the procedure with the child receiving prostaglandin E1 infusion—for three reasons: first, the infant is in a more stable hemodynamic state during the procedure. Second, a left-to-right ductal shunt maintains pulmonary blood flow during balloon occlusion of the RVOT. Finally, the presence of a patent ductus arteriosus permits the exchange guidewire to be positioned across the pulmonary valve and into the descending aorta, a course that facilitates catheter exchanges and subsequent valve dilation. The authors have reported the results of balloon valvuloplasty attempted in 12 infants with critical pulmonary stenosis (n = 10) or membranous pulmonary atresia with intact ventricular septum (n = 2).20 These infants ranged in age from 1 to 38 days and in weight from 2.9 to 4.5 kg. Nine were receiving a prostaglandin E1 infusion. In one child with critical pulmonary stenosis and a diminutive right ventricle, the RVOT was perforated during an attempt to cross the valve. This child was taken to the operating room, where the perforation was oversewn and a Blalock-Taussig shunt performed. In the remaining 11 infants, balloon valvuloplasty was successfully performed using balloons ranging in diameter from 2.5 to 12 mm. In patients with membranous pulmonary atresia, the membrane can be perforated with a guidewire or a radiofrequency wire followed by balloon dilation (Fig. 54-8). If the infant remains significantly hypoxemic, additional pulmonary blood flow can be provided by percutaneous stenting of the ductus arteriosus (Fig. 54-9).
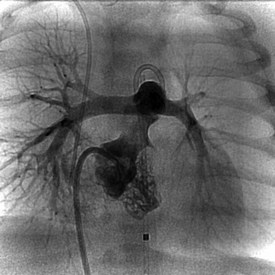
Figure 54-8 Anteroposterior right ventricular angiogram in the same infant as Figure 54-7, after radiofrequency perforation and dilation of membranous pulmonary valve atresia. The patent RV outflow tract is now evident, with good-size distal pulmonary arteries.
Adults
Several reports have described the successful application of percutaneous balloon valvuloplasty for treatment of adults with pulmonary valve stenosis.14,18,22–32 Table 54-2 summarizes the pertinent clinical and hemodynamic data from 14 publications (including this report) describing the acute results of pulmonary valvuloplasty in adolescents and adults. Pulmonary valvuloplasty has been performed successfully in patients as old as 84 years. In most published cases, a single-balloon technique has been used. When a 20-mm-diameter balloon is insufficient, however, the double-balloon technique has usually been necessary. In these reports, balloon valvuloplasty acutely reduced the peak systolic gradient by an average of 60% to 65%, from a range of 53 to 260 mm Hg before the procedure to 2 to 90 mm Hg after valvuloplasty. In most cases, the peak systolic gradient immediately after valvuloplasty was in the mild range (20 to 40 mm Hg). For example, Al Kasab and colleagues18 reported the effects of valvuloplasty in 12 adults, ranging in age from 21 to 37 years, with valvar pulmonary stenosis. In these patients, valvuloplasty acutely reduced the peak systolic gradient from 86 to 28 mm Hg. Transient ventricular arrhythmias were noted in 30% of patients, but no serious complications were described. Similarly, Fawzy and colleagues25 described 8 adult patients with valvar pulmonary stenosis in whom percutaneous balloon valvuloplasty reduced the peak systolic gradient from 107 to 36 mm Hg. Thus, available data clearly indicate that percutaneous balloon valvuloplasty provides effective therapy in adults as well as in children with congenital pulmonary valve stenosis. Balloon valvuloplasty appears to be effective even in the oldest patients, in whom valve calcification may be present.22
Long-Term Studies
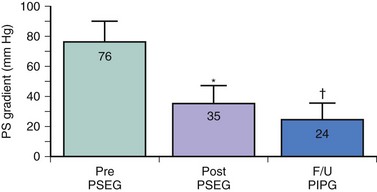
Long-term studies of balloon pulmonary valvuloplasty have confirmed that the benefits of this procedure are durable and comparable to the results of surgical valvotomy. McCrindle and Kan33 reported the long-term results of balloon valvuloplasty performed between 1981 and 1986 in 42 patients (median age 4.6 years), for whom follow-up data beyond 2 years were available. Balloon valvuloplasty acutely reduced the peak systolic gradient from 70 to 23 mm Hg; at long-term follow-up more than 2 years after the procedure, the Doppler-predicted gradient was 20 mm Hg. Doppler peak instantaneous gradients were less than 36 mm Hg at long-term follow-up in 86% of patients. The authors found age younger than 2 years at the time of balloon valvuloplasty to be a risk factor for late follow-up gradients exceeding 36 mm Hg. Long-term data from the Pediatric Valvuloplasty Registry were reported on 533 patients up to 8.7 years after balloon pulmonary valvuloplasty.34 A total of 84 patients (16%) required either a surgical valvotomy or a repeat balloon dilation. Of the 449 patients who had not undergone a repeat procedure, 399 had mild residual stenosis (<36 mm Hg), 36 had residual stenosis >36 mm Hg, and the late gradient was unknown in 14. Independent risk factors for a suboptimal late outcome included small valve annulus diameter, higher early residual gradient, smaller balloon-annulus diameter ratio, and earlier year at initial intervention. We assessed the long-term (4 to 5 years) outcome after balloon pulmonary valvuloplasty in childhood and compared the results to a matched surgical control group.35 Follow-up data obtained in 20 children 4 to 7.8 years after balloon valvuloplasty documented excellent late results without significant restenosis. The peak systolic gradient measured at cardiac catheterization in these children averaged 76 mm Hg before and 35 mm Hg immediately after balloon valvuloplasty. At long-term follow-up, the Doppler peak instantaneous gradient was 24 mm Hg, or significantly less than that measured by catheterization immediately after the procedure (Fig. 54-10). Pulmonary valve insufficiency was mild in 9 of 20 patients and absent in the remainder. Twenty-four-hour Holter monitoring documented only grade 1 ventricular ectopic activity in one patient and none in the remaining 19 patients. Comparison to the matched surgical control group demonstrated that, although the residual gradient was slightly less after surgery (16 vs. 24 mm Hg; P = 0.01), the surgical group had significantly more pulmonary valve insufficiency and late ventricular arrhythmias. Late follow-up data, therefore, document excellent long-term results after percutaneous pulmonary balloon valvuloplasty and support the use of this procedure as treatment of choice for patients with isolated valvar pulmonary stenosis. A recent study of exercise capacity in 41 patients a median of 13.1 years after pulmonary balloon valvuloplasty showed that peak oxygen consumption was mildly below the expected value in this group (92 ± 17% of predicted, P = 0.006).36 Cardiac magnetic resonance imaging in these patients showed pulmonary regurgitation fractions of 1% to 45%, with a median of 10%. Moderate regurgitation (fraction 31% to 49%) was present in 7 patients (17%), with mild regurgitation (fraction 16% to 30%) in 7 more patients. The 14 patients with mild or moderate pulmonary regurgitation had significantly lower peak oxygen consumption than those with lesser amounts of regurgitation (85 ± 17% vs. 96 ± 16% of predicted, P = 0.03). Right ventricular dilation (end-diastolic volume z-score >2) was present in 40% of the study group, and indexed end-diastolic volume of the right ventricle correlated with pulmonary regurgitation fraction (R = 0.79, P < 0.001). These results suggest that relatively modest amounts of pulmonary regurgitation may result in long-term right ventricular dilation and reduced exercise tolerance. Alternatively, these results may simply reflect the severe nature of the pulmonary stenosis in these patients prior to intervention and the aggressive balloon dilation procedures required (median age at intervention 0.2 years, median right-ventricle-to-aorta pressure ratio 110% before valvuloplasty, median balloon-to-annulus diameter ratio 1.3, range 1.0 to 2.0).
Complications
Beyond infancy, percutaneous balloon pulmonary valvuloplasty is a very safe procedure. In the Pediatric Valvuloplasty Registry, the only two deaths occurred in infants with critical pulmonary stenosis, and the single case of perforation and tamponade occurred in an 8-day-old neonate.13 Minor complications were primarily related to vascular injury or hemorrhage and were also much more common during the first 12 months of life. Overall, the Pediatric Valvuloplasty Registry noted a 1.2% to 1.8% frequency of major complications and a 4.8% frequency of minor complications in 168 infants. In contrast, in 656 children and adults, the frequency of major complication was 0.8% and the frequency of minor complication was 1.7%. Premature ventricular beats and right-bundle-branch block occur commonly during the procedure owing to catheter and wire manipulation within the right ventricle, but there have been no reports of long-term arrhythmias after valvuloplasty. Valvuloplasty may cause injury to the femoral vein, especially when the procedure is performed in infancy. Finally, the mild pulmonary valve insufficiency commonly seen after pulmonary valvuloplasty, while perhaps not entirely benign,36 is rarely of clinical importance and may be less severe than after surgical valvotomy.35
Conclusions and Recommendations
Percutaneous balloon pulmonary valvuloplasty is the treatment of choice for children and adults with isolated congenital valvar pulmonary stenosis. Valvuloplasty successfully reduces significant RVOT obstruction, with a residual gradient that is usually in the trivial to mild range (i.e., <30 mm Hg). Follow-up studies have documented long-term effectiveness, with little restenosis as late as 9 years after the procedure. In our opinion, pulmonary valvuloplasty is indicated in patients with isolated pulmonary valve stenosis whose resting peak systolic pressure gradient exceeds 40 mm Hg in the presence of a normal cardiac output. The procedure is effective in neonates, children, and adults as old as 84 years.22 Unless the valve annulus is severely hypoplastic, patients with a calcified or dysplastic pulmonary valve may also derive significant hemodynamic benefit from balloon valvuloplasty.
 Aortic Balloon Valvuloplasty
Aortic Balloon Valvuloplasty
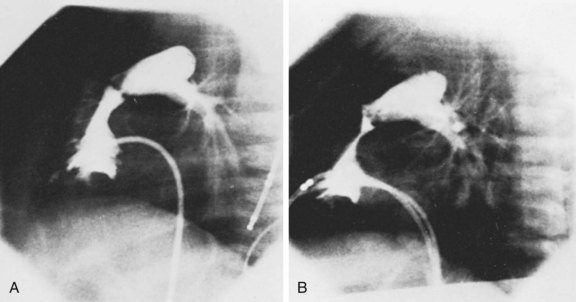
Aortic valve stenosis accounts for 4% to 6% of all cases of congenital heart disease.37 Left ventricular outflow tract (LVOT) obstruction elicits left ventricular hypertrophy and myocardial fibrosis, which may eventually lead to left ventricular dysfunction and congestive heart failure. Unlike most cases of congenital pulmonary valve stenosis, congenital aortic stenosis tends to progress over time.38 Nevertheless, intervention usually is not indicated unless the degree of LVOT obstruction is severe (catheter gradient >65 mm Hg) or there is associated left ventricular dysfunction, heart failure, ischemia, or symptoms of angina, syncope, or presyncope. This recommendation is based on the fact that all current forms of therapy for aortic valve stenosis are palliative in nature. Surgical valvotomy, widely regarded in the past as the initial treatment of choice for congenital aortic valve stenosis, is associated with a high incidence of late (5 to 20 years) restenosis.39 Prosthetic aortic valve replacement is associated with risks of thromboembolic complications and risks associated with anticoagulation therapy that may be considerable in young, active patients. Thus, because current treatment options are not curative, intervention for congenital aortic valve stenosis is usually delayed until clear indications exist. Percutaneous balloon valvuloplasty for the treatment of congenital valvar aortic stenosis in children was first described in 1984.40 Balloon valvuloplasty typically reduces the LVOT obstruction to the mild range and is the treatment of choice for children with congenital aortic stenosis who require intervention. The effectiveness of balloon dilation relates to the underlying morphological substrate. Most congenitally stenotic aortic valves are bicuspid, involving a single central or eccentric commissure with a variable degree of fusion of its edges. The valve leaflets themselves are thickened but are rarely calcified in childhood (Fig. 54-11). In older patients and in children with prior valve surgery, the leaflets may calcify, becoming less mobile and less amenable to balloon dilation. In congenital aortic valve stenosis as in pulmonary valve stenosis, balloon valvuloplasty reduces the degree of stenosis by separating valve leaflets along the lines of commissural fusion (Fig. 54-12). Because the valve leaflets are typically supple in younger patients and the obstruction to ventricular outflow relates primarily to incomplete cusp separation during systole, balloon dilation provides substantial hemodynamic improvement in these cases. This is in marked contrast to older patients with calcific aortic stenosis, in whom balloon valvuloplasty has proven to be much less successful.41 In these patients the aortic valve stenosis is acquired, primarily as a result of calcium deposition within the leaflets, and little or no commissural fusion is present.42,43 Therefore differences in valve morphology, and thus in the mechanism by which balloon dilation improves valve function, explain the observation that balloon valvuloplasty is effective in younger patients with congenital aortic stenosis but typically ineffective in adults with calcific aortic stenosis.
Successful percutaneous balloon valvuloplasty in children with congenital aortic valve stenosis was first reported in 1984 by Lababidi and colleagues.40, 44 Since then the effectiveness of balloon valvuloplasty in children and adolescents with congenital aortic valve stenosis has been clearly demonstrated.45–53 The procedure usually reduces the peak systolic gradient by approximately 60%, and severe aortic regurgitation is uncommon. Vascular complications have been limited primarily to neonates and young infants and have diminished in recent years with the development of smaller-profile valvuloplasty catheters.
Technique
Percutaneous aortic valvuloplasty is usually performed from a retrograde transarterial approach, although the antegrade transseptal approach can also be used. We prefer the retrograde approach, using a transseptal catheter for continuous left ventricular pressure monitoring throughout the procedure. After the transseptal puncture is accomplished, heparin is administered to increase the activated clotting time (ACT) to approximately 250 to 300 seconds. The aortic stenosis gradient is measured before angiography from simultaneous ventricular and aortic pressure recordings. In our center, the criteria for performing aortic balloon dilation include (1) a peak systolic pressure gradient at rest of 65 mm Hg or more, (2) a peak systolic gradient of 50 to 64 mm Hg in association with symptoms or ischemic changes on electrocardiography, or (3) patients with low cardiac output regardless of measured pressure gradient. A modest aortic stenosis gradient is typical in infants with critically severe aortic stenosis in whom left ventricular failure and shock are present. Once the decision to proceed with balloon valvuloplasty has been made, the correct balloon diameter must be selected. Proper balloon size depends on aortic valve annulus diameter, which can be measured by echocardiography or by angiography. If a single-balloon technique is used, a balloon is chosen whose diameter is equal to or 1 mm smaller than 90% to 100% of the diameter of the aortic valve annulus. If a double-balloon technique is used, we use two balloons of similar diameter whose sum is 1.2 to 1.3 times the diameter of the single-balloon diameter thought to be optimal for the patient.46 We prefer the double-balloon technique in patients whose aortic valve annulus exceeds 25 mm or in those whose aortic valve annulus is large for their body size so as to minimize the size of the balloon catheter and arterial sheath required for the procedure. Unlike the case in pulmonary balloon valvuloplasty, oversized balloons are not used for aortic valvuloplasty because these have been shown to increase the risk of injury to the aortic valve and annulus.47 The balloon or balloons are inflated by hand (i.e., a manometer is not used) until the waist produced on the balloon by the valve is relieved (Fig. 54-13). Balloon inflation is kept as brief as possible to minimize arterial hypotension during the procedure. Because the inflated balloon may be ejected across the aortic valve, several balloon inflations may be required, with minor adjustments in position to ensure that the valve is adequately dilated. Repeat simultaneous measurements of left ventricular and aortic pressures are made to quantify the residual aortic stenosis gradient. An aortic root angiogram is performed to detect any aortic insufficiency that may have been produced by the procedure.
Acute Results
In most patients with congenital aortic valve stenosis, balloon valvuloplasty provides relief of LVOT obstruction (Fig. 54-14) comparable to the results of surgical valvotomy.38,54 The expected peak systolic ejection gradient across the aortic valve after the procedure is approximately 20 to 40 mm Hg.45–52 If a technically adequate balloon dilation fails to achieve a satisfactory hemodynamic result in a patient with congenital aortic valve stenosis, a more complex diagnosis is suggested; such patients may be found to have annular hypoplasia or valve leaflet calcification. The largest series of balloon aortic valvuloplasty procedures for congenital aortic stenosis was reported by the Pediatric Valvuloplasty Registry.45,52 The acute results of 630 balloon valvuloplasty procedures were reported in 606 children ranging in age from 1 day to 18 years who underwent the procedure at 23 institutions between 1984 and 1992. Overall, the procedure resulted in an immediate decrease in peak systolic pressure gradient across the aortic valve by 60%. In the initial registry report of 204 children,45 most of the acute complications and deaths occurred in newborns. Five deaths were reported (mortality rate, 2.4%), four of which occurred in newborns and one in a 3-month-old child. Two deaths were related to aortic rupture, two due to aortic valve trauma, and one occurred as a result of a torn iliofemoral artery. There was no mortality in patients older than 3 months of age. The larger report52 evaluated predictors of a suboptimal outcome, which was defined as failure to perform the valvuloplasty (which occurred in 4.1%), an immediate residual gradient of 60 mm Hg or more, a left ventricular systolic pressure exceeding aortic systolic by 60% or more, mortality, or major morbidity. Overall, a suboptimal outcome was reported for 17% of the 630 valvuloplasty procedures. The identified independent predictors for a suboptimal outcome included age younger than 3 months, a greater predilation systolic gradient, a balloon-annulus diameter ratio under 0.9, the coexistence of an unrepaired coarctation, and an earlier date of procedure. Three months appeared to be a significant threshold below which the procedural outcome was adversely affected. Patients younger than 3 months were more likely to experience failure to perform the procedure (15.7% vs. 1.7%), suboptimal residual stenosis (17.8% vs. 7.5%), major morbidity (16.7% vs. 4.1%), and mortality (8.3% vs. 0.6%). The effect of the balloon-annulus diameter ratio was thoroughly evaluated, and the optimal ratio was found to be between 0.90 and 0.99. Smaller ratios were associated with an increased risk of suboptimal gradient relief. Larger-diameter ratios were associated with a greater risk of aortic insufficiency after valvuloplasty. The authors’ experience with percutaneous balloon aortic valvuloplasty was similar. Between 1985 and 1992, balloon aortic dilation was performed in 68 children, adolescents, and young adults with congenital aortic valve stenosis (Table 54-3). Patients ranged in age from 1 day to 24 years (8.7 ± 6.9 years) and in weight from 2.1 to 120 kg (35.7 ± 27.7 kg). The valvuloplasty procedure was performed with balloon diameters ranging from 5 to 20 mm, and the double-balloon technique was used in 36 patients. Percutaneous balloon valvuloplasty acutely decreased the peak systolic aortic stenosis gradient by 56%, from 77 ± 22 to 34 ± 16 mm Hg. Left ventricular systolic pressure decreased acutely from 171 ± 31 to 133 ± 25 mm Hg, and left ventricular end-diastolic pressure decreased slightly from 14.5 ± 4.3 to 11.9 ± 5.7 mm Hg. There was no change in cardiac output or heart rate after the dilation procedure. In 52 of 68 patients (76%), there was no increase in the degree of aortic insufficiency from that present before balloon valvuloplasty. An increase of 1 grade (on a scale of 0 to 4+ ) occurred acutely in 10 patients (15%), and an increase of 2 grades occurred in 6 patients (9%). No patient required emergent or urgent surgical intervention because of valvuloplasty-induced aortic insufficiency. In our series, we found no relationship between the effectiveness of balloon valvuloplasty and patient age or size. We have noted, however, that patients with an unsatisfactory degree of gradient relief (residual gradient >45 mm Hg) have had more complex disease, including annular hypoplasia or valve leaflet calcification. In this series, there has been no patient with unsatisfactory gradient relief from balloon valvuloplasty who subsequently underwent a successful surgical valvotomy. Such patients, instead, have required more complex surgical intervention, including prosthetic aortic valve replacement or the Konno operation.
TABLE 54-3 Pertinent Data before and after Balloon Aortic Valvuloplasty in 68 Patients
| Number | 68 |
| Age (years) | |
| Mean ± SD | 8.7 ± 6.9 |
| Range | 1 d–24 yr |
| Weight (kg) | |
| Mean ± SD | 35.7 ± 27.7 |
| Range | 2.1–120 |
| Systolic gradient (mm Hg) | |
| Before | 77 ± 21 |
| Immediately after | 34 ± 16 |
| Left ventricle systolic pressure (mm Hg) | |
| Before | 171 ± 31 |
| Immediately after | 133 ± 25 |
| Cardiac index (L/min/m2) | |
| Before | 3.64 ± 0.95 |
| Immediately after | 3.35 ± 0.80 |
Infants
Infants with critical aortic stenosis typically present in severe congestive heart failure and shock, with profound left ventricular dysfunction (Fig. 54-15). At times it can be difficult to distinguish neonatal critical aortic stenosis from hypoplastic left heart syndrome, as the aortic valve annulus, mitral valve annulus, and even the left ventricular length can be undersized. In addition, the aortic valve leaflets (or leaflet) often exhibit a markedly dysplastic appearance on echocardiography, which in an older child would indicate a low likelihood of successful balloon valvuloplasty. Despite these real or apparent obstacles to success, balloon aortic valvuloplasty has proved to be remarkably successful, with results comparable to surgical intervention at several premier institutions.55–58 The clinical status of these patients generally leaves little room for doubt that urgent intervention is required, but the choice between relief of aortic stenosis and univentricular palliation is not always obvious. Unlike critical pulmonary stenosis, where placement of a systemic to pulmonary artery shunt can compensate for inadequate support of the pulmonary circulation by hypoplasia of right heart structures, patients with left heart structures inadequate to support the systemic circulation face death unless a successful Norwood palliation can be performed. Several systems have been developed for predicting which neonates have the potential for adequate systemic perfusion after relief of aortic valve stenosis, the most popular of which was reported by Rhodes and colleagues.59 The child should be stabilized with prostaglandin E1 infusion and intravenous inotropic support prior to the procedure, with extracorporeal life support if necessary. If possible, we use the transumbilical approach (Fig. 54-16) to spare the infant’s femoral artery (which may be required for future percutaneous valve dilation procedures). The carotid artery60 and transvenous antegrade61 approaches have also been reported as means to avoid femoral artery injury in newborn infants. We have tended to employ a single balloon, typically 6 to 7 mm in diameter, aiming for a single inflation. In contrast, McElhinney and colleagues57 begin with smaller balloons and perform serial dilations with progressively larger balloons until the desired degree of relief is obtained. The Congenital Heart Surgeons Society reported the results of intervention in 110 neonates with critical aortic stenosis from 18 institutions.56 Balloon aortic valvuloplasty was the initial procedure in 82 patients and surgical valvotomy in the remaining 28. Relief of aortic stenosis was significantly better in the balloon valvuloplasty group (gradient reduction of 65 ± 17%, median residual gradient 20 mm Hg, vs. 41 ± 32%, median residual gradient 36 mm Hg), although there was also a trend toward more aortic insufficiency in this group. Early mortality was 18%, with no difference between groups. In our own study,55 30 neonates were assigned to balloon valvuloplasty and 17 to surgical intervention on an intent-to-treat basis; early mortality was 13% in both groups. Early mortality was 14% in a series of 113 neonates treated with balloon aortic valvuloplasty, with about one-third of survivors requiring repeat intervention within 1 year.57 Echocardiographic estimates of valve thickness or mobility have not correlated with valvuloplasty success in neonates.
Fetal Intervention
Balloon valvuloplasty has been proposed for fetuses with aortic stenosis for the purpose of preventing them from developing hypoplastic left heart syndrome. Research has focused on accurately identifying fetuses at risk for this progression, developing techniques for in utero intervention, reducing the risks of technical failure and of fetal demise, and on predicting which fetuses are most likely to benefit from intervention. The Harvard group has reported its experience with 70 fetuses from March 2000 through October 2008.62 Valvuloplasty was attempted in midgestational fetuses who met criteria for aortic stenosis with evolving hypoplastic left heart syndrome, and all were thought to have a potentially salvageable left ventricle. Technical success was achieved in 52 procedures (74%), and 61 pregnancies resulted in live birth at a viable gestational age (87%). Of the 47 infants born alive at a viable gestational age after a technically successful midgestational valvuloplasty, 20 (43%) have achieved a biventricular circulation and 27 progressed to hypoplastic left heart syndrome despite intervention. Based on this experience, the group has modified its selection criteria to require unequivocal demonstration of aortic stenosis (i.e., elimination of any candidates with possible aortic atresia) and a minimum left ventricular long-axis z-score of −2. In addition, candidates must qualify under at least four of the following five criteria: left ventricular long-axis z-score >0; left ventricular short-axis z-score >0; aortic valve annulus z-score >−3.5; mitral valve annulus z-score >−2; Doppler-estimated mitral regurgitation or aortic stenosis maximum systolic gradient ≥20 mm Hg. Of the fetuses who met these criteria in retrospect, technical success was achieved in 87%, with biventricular circulation in 50% of those born alive at a viable gestational age after successful valvuloplasty. These results warrant further investigation.
Young Adults
Balloon aortic valvuloplasty in young adults with congenital aortic valve stenosis yields results similar to those in children with the same disease and in patients with rheumatic aortic stenosis, all of whom exhibit commissural fusion to a significant degree. In contrast, balloon valvuloplasty is of limited utility in patients with degenerative or calcific aortic stenosis.41,63–65 We have reported our experience with balloon valvuloplasty in 15 young adult patients, aged 15 to 24 years, with congenital aortic valve stenosis.53 All were judged to have severe disease. The valve annulus ranged from 18.5 to 30 mm in diameter. Balloons were used ranging in diameter from 10 to 20 mm, and the double-balloon technique was used in 12 patients. In one patient with access to only one femoral artery available, the double-balloon technique was performed using a single retrograde balloon together with a balloon placed anterograde through a transseptal puncture. In these 15 young adults, balloon valvuloplasty acutely reduced the peak systolic aortic valve gradient from 73 to 35 mm Hg. Left ventricular systolic pressure decreased from 179 to 147 mm Hg without an associated change in cardiac output. Aortic insufficiency was unchanged by the procedure in 9 patients, increased by one grade in 4, and increased by two grades in 2. An unsatisfactory result was obtained in 3 patients, in whom the residual systolic gradient was 70 mm Hg or more. Two of these patients had mild annular hypoplasia (18.5 and 19 mm), and the third had moderate valve leaflet calcification (related to prior surgical valvotomy in childhood). All three of these patients have required prosthetic aortic valve replacement. The remaining 12 patients have done well during intermediate-term follow-up. Eight underwent elective follow-up cardiac catheterization 1 to 2.5 years after balloon valvuloplasty, which documented no restenosis. In these patients, the follow-up peak systolic gradient at cardiac catheterization was 30 mm Hg, and there was no change in the degree of aortic regurgitation. Although this is a small series, the data lead us to believe that percutaneous balloon valvuloplasty should be attempted in young adults with congenital aortic valve stenosis unless the valve annulus is hypoplastic or the valve is calcified.
Long-Term Studies
Percutaneous balloon valvuloplasty for congenital aortic stenosis should be regarded as a palliative therapeutic procedure. As is the case after surgical aortic valvotomy,39 late restenosis (5 to 20 years) should be expected after a successful balloon dilation procedure. We would warn, however, against comparing follow-up peak instantaneous gradients determined by Doppler echocardiography against catheter-based measurements of peak systolic gradient obtained immediately after valvuloplasty.66 Because Doppler peak instantaneous and catheter peak systolic gradients may differ substantially, particularly in patients with aortic insufficiency, a false impression of restenosis may be obtained in comparing Doppler with catheter gradient measurements. The intermediate-term effectiveness of balloon valvuloplasty in children with congenital aortic stenosis was prospectively evaluated in our center.67 A follow-up cardiac catheterization was performed in 27 of the first 30 children to undergo successful percutaneous balloon dilation at our institution between 1985 and 1988 an average of 1.7 years (0.8 to 3.8 years) after balloon valvuloplasty. No restenosis was documented in these patients, with the greatest increase in peak systolic gradient at recatheterization being 14 mm Hg. In this group of 27 children (mean age, 8.6 years), balloon valvuloplasty acutely decreased the peak systolic gradient from 76 to 31 mm Hg. At follow-up 1.7 years later, the peak systolic gradient remained 29 mm Hg (Fig. 54-17). At the follow-up cardiac catheterization, 20 of 27 patients (74%) had no increase in the degree of aortic insufficiency that had been present before balloon valvuloplasty. In the 7 patients in whom balloon dilation resulted in increased valve insufficiency, 5 had a 2+ increase in aortic insufficiency and 2 had a 1+ increase in aortic insufficiency at the follow-up study (compared with prevalvuloplasty insufficiency). The degree of the aortic insufficiency after valvuloplasty remained stable during the follow-up period in 4 of these 7 patients and increased 1 to 2 grades in the remaining three patients. Galal and colleagues reported the 3- to 9-year follow-up of 26 patients who had balloon aortic valvuloplasty at ages ranging from 6 weeks to 20 years.61
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree