The purpose of the present study was to assess the results and technical aspects of attempted transcatheter recanalization of occluded pulmonary arteries or major systemic veins from our center. Occluded pulmonary arteries or major systemic veins are often not considered amenable to transcatheter treatment and can be a cause of significant hemodynamic compromise for patients. The records of patients with occluded pulmonary arteries or major systemic veins who underwent cardiac catheterization from April 1997 to February 2011 were reviewed. We identified 18 patients, of whom, 9 had occluded pulmonary arteries and 9 occluded systemic veins. Recanalization was achieved in 17 of the 18 patients (94%). At a median follow-up of 35 months (range 6 months to 12 years), all vessels remained patent. The freedom from reintervention rate in the entire cohort was 61% at 6 months, 35% at 1 year, and 35% at 5 years. Freedom from an unplanned reintervention was 67% at 6 months. Procedural adverse events occurred in 2 patients. One death occurred within 24 hours of the catheterization procedure and 2 late deaths occurred, all unrelated to the procedure. In conclusion, both acute and chronic success at recanalizing the occluded pulmonary arteries and major systemic veins can be achieved using percutaneous techniques. Careful follow-up is needed, because reintervention can be necessary to maintain long-term vessel patency without stenosis.
Placement of central lines, cardiac catheterization, and surgical procedures can result in occlusion of pulmonary arteries or major systemic veins, particularly in low-flow states. Traditionally, surgical therapy for these lesions has been thought to be the mainstay of treatment. However, these surgical procedures are often complex and are also associated with a significant risk of repeat occlusion of the reconstructed vessel segment. Moreover, if the occlusion is recognized acutely, as it often is, re-establishing vessel patency surgically might not be the ideal modality of treatment in the early postoperative period for patients who might be hemodynamically unstable. Transcatheter treatment of many congenital heart lesions has emerged as an alternative to surgical therapy. The purpose of the present study was to review our experience of recanalization of totally occluded proximal pulmonary arteries and major systemic veins in a heterogeneous population of patients with congenital heart disease as an alternative therapy to surgical reconstruction. Specifically, we sought to assess the acute success rates of recanalization of these vessels and the intermediate effects of maintaining vessel patency.
Methods
This was a retrospective and descriptive study of patients in whom an attempt was made to recanalize a totally occluded pulmonary artery or major systemic vein in the catheterization laboratory from April 1997 and February 2011. The database of the Center for Pediatric and Congenital Heart Disease at the Children’s Hospital (Cleveland Clinic, Cleveland, Ohio) was queried for patients who had undergone attempts at transcatheter recanalization of major intrathoracic vessels, using the terms “occlusion,” “recanalization,” and “complete obstruction.” Only native or partially surgically augmented pulmonary arteries and major systemic veins (i.e., superior vena cava, inferior vena cava, and innominate veins) were included in the present study. Occluded vessels in which a previous catheter-based intervention (i.e., occlusions within previously placed stents) were excluded. The study was performed in accordance with the Cleveland Clinic’s institutional review board’s guidelines and policy.
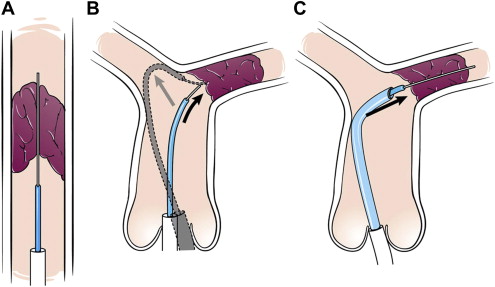
We have previously described the technical aspects of procedures to re-establish vessel patency of occluded intrathoracic vessels and pathways. In brief, all procedures were performed with the patient under general anesthesia in the cardiac catheterization laboratory using biplane cineangiography. The presence of a “beak” was identified as a target for crossing the occluded vessel segment. When the distal vessel segment could be accessed at the same time as the segment proximal to the occlusion (i.e., by way of a systemic-to-pulmonary artery shunt), simultaneous angiograms were performed to aid in crossing the occluded segment. Alternatively, a snare catheter was placed in the distal segment to serve as a target. If the distal vessels could not be directly accessed, they were visualized by indirect methods using wedge angiograms or aortograms. Attempts were made initially to cross vessels with the soft ends of standard guidewires or hydrophilic guidewires. When that was not feasible, the stiff ends of guidewires or transseptal needles (Brockenbrough needle, Medtronic, Minneapolis, Minnesota) were used to cross the occluded vessel segment. For the purpose of the present study, the stiff ends of the guidewires and relatively stiff guidewires (i.e., V-18 Control Wire Guide Wires, Boston Scientific, Natick, Massachusetts) were both considered to be stiff guidewires. If a relatively straight course was not present, a modified guide catheter was used to provide support for the penetrating implement ( Figure 1 ). A hydrophilic glide catheter (Terumo Glidecath, Terumo Medical, Somerset, New Jersey) and hydrophilic guidewire (Terumo glidewire, Terumo Medical), “guide and glide system” was then used to penetrate the occluded segment. If the occluded segment could not be crossed with a guidewire, a transseptal needle was advanced through the modified guide catheter or a sheath. Radiofrequency perforation was attempted briefly in 1 patient; however, the involved vessel was eventually crossed with a transseptal needle.
Specific anticoagulation regimens were not applied before or after the procedures. Anticoagulation regimens (i.e., warfarin, aspirin, and/or heparin) varied, depending on the lesion and physician preference. In general, after a successful recanalization procedure, the patients received anticoagulation therapy for a minimum of 6 months after the procedure if a stent had been placed or in low-flow states after angioplasty alone was performed.
Continuous measures were described using the mean ± SD. Analyses were performed using SAS software, version 9.1, (SAS Institute, Cary, North Carolina) and R software, version 2.12 (R Programming, Vienna, Austria). The duration of vessel occlusion was defined as the interval from the first documentation of a plausible inciting event (i.e., surgery) to the diagnosis of vessel occlusion. Alternatively, if a patient developed symptoms from the occlusion, the interval from symptom development to documentation of occlusion was used. In the present report, acute occlusions were defined as occlusions <3 months old. Unplanned interventions were defined as interventions that occurred sooner than intended because of symptoms or documentation on noninvasive imaging of restenosis or reocclusion. Planned interventions were defined as interventions that were performed to account for vessel growth. Chronic success was defined as patency >6 months of the initial procedure.
Results
Cardiac catheterization involving attempted recanalization of an occluded pulmonary artery or major systemic vein was attempted in 18 patients. The baseline demographics, mechanism of occlusion, vessel characteristics, and procedural specifics are listed in Table 1 . Symptoms were present in 3 of 9 of the patients with pulmonary artery occlusions (cyanosis) and 7 of 9 patients with systemic vein occlusions (edema and/or fatigue in 6 patients, neurologic event due to a right-to-left shunt in 1 patient). The overall fluoroscopy and procedure times were 51 minutes (range 25 to 109) and 221 minutes (range 124 to 417).
| Vessel | Pt. No. | Gender (M/F) | Age (y) | Weight (kg) | Diagnosis | Presumed Etiology | Occlusion Time (mo) | Occlusion Length (mm) | Recanalization Method | Maximum Balloon (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary arteries | Total patients: 9 | 5/4 | 1.1 (0.3–7.8) | 6.4 (4.9–18.5) | 1.2 (0.2–84.6) | 11.7 ± 7.4 | 6.3 ± 1.9 | |||
| Neo-LPA | 1 | 0.6 | 6.4 | HLHS | Post-Glenn: neo-aortic compression | 2 | 13.2 | Hydrophilic wire | 7 | |
| Neo-LPA | 2 | 0.3 | 4.9 | HLHS | Post-Glenn: neo-aortic compression vs thrombus | 1.2 | 10.2 | Stiff wire | 6 | |
| Neo-LPA | 3 | 5.4 | 17.2 | HLHS | Post-Glenn: thrombus | 0.5 | 10 | Hydrophilic wire | 6 | |
| Neo-LPA | 4 | 0.5 | 5.8 | HLHS | Post-Glenn: thrombus | 0.2 | 5.5 | Hydrophilic wire | 8 | |
| Neo-LPA | 5 | 7.8 | 17.3 | HLHS | Post-Glenn: neo-aortic compression | 84.6 | 30 | Transseptal needle | 6 | |
| Neo-LPA | 6 | 7.8 | 18.5 | DOLV | Post-Glenn: neo-aortic compression vs thrombus | 83.3 | 9 | Stiff wire | 10 | |
| Neo-LPA | 7 | 0.4 | 6.2 | TA/PA | Post-Glenn: thrombus | 0.3 | 12.5 | Hydrophilic wire | 5 | |
| LPA | 8 | 1.1 | 6 | TOF/PA, VSD, MAPCAs | Postoperative: thrombus | 0.4 | 5.5 | Hydrophilic wire | 3 | |
| LPA | 9 | 1.4 | 10.3 | TOF | Postoperative: compression from MPA | 2 | 9 | Hydrophilic wire | 6 | |
| Systemic veins | Total patients: 9 | 8/1 | 9.7 (0–34) | 23.4 (1.3–129) | 27.5 (0.1–384) | 18.2 ± 6.2 | 10.5 ± 5.2 | |||
| SVC | 10 | 34 | 74.1 | d-TGA | SVC obstruction after Mustard procedure | 384 | 18.3 | Transseptal needle | 16 | |
| SVC | 11 | 13.6 | 87.1 | d-TGA | SVC obstruction after Mustard procedure | 2.7 | 16.9 | Transseptal needle | 12 | |
| SVC | 12 | 0 | 3.7 | d-TGA | SVC obstruction after bypass for arterial switch | 0.1 | 26 | Stiff wire | 7 | |
| SVC | 13 | 9.7 | 23.4 | DORV after bilateral BDGs | Post-Glenn: thrombus | 63.5 | 22 | Transseptal needle | 15 | |
| SVC | 14 | 6.8 | 17.1 | PA/VSD, MAPCAs | Thrombus from bypass cannulation | 47 | 10 | Transseptal needle | 12 | |
| SVC | 15 | 0.1 | 1.3 | Extreme prematurity | Thrombus from PICC line | 0.1 | 24 | Unsuccessful | N/A | |
| Innominate vein | 16 | 0.7 | 8.5 | HLHS | Thrombus from bypass cannulation | 7.9 | 23.6 | Stiff wire | 4 | |
| Innominate vein | 17 | 22.4 | 76.8 | Systemic pulmonary venous collateral | Cryptogenic brain abscess led to diagnosis | NA | 10.6 | Stiff wire | 8 | |
| SVC | 18 | 22.9 | 129 | ASD, subaortic stenosis | Postoperative thrombus | 132 | 12 | Hydrophilic wire | 10 |
For the pulmonary artery group, a significant difference was seen between the left and right pulmonary artery diameters before the Glenn operation or repair for tetralogy of Fallot (2.1 ± 1.6 mm vs 6.4 ± 1.9 mm, respectively). In patients undergoing Glenn operations, all but 1 underwent Norwood stage 1 procedures using a Blalock-Taussig shunt. In the 1 patient who underwent Norwood stage 1 procedure with a Sano modification and 1 patient who had a previous Blalock-Taussig shunt with tetralogy of Fallot, left pulmonary artery stenosis was induced by these procedures.
Acute success was achieved in 17 of 18 vessels (94%; Figure 2 ). Recanalization of these occluded vessels was achieved with a variety of penetrating implements ( Table 1 ). The unsuccessful procedure occurred in a 2-month-old premature infant (patient 15) with chylous pleural effusions related to a long-segment occlusion of his superior vena cava. Occlusions were crossed with hydrophilic guidewires in 6 of 9 patients with acute occlusions compared to only 1 of 7 patients with chronic occlusions (1 procedure unsuccessful, and occlusion time not discernable in 1 patient). Primary angioplasty was performed in 5 patients, with primary stenting in 12 ( Figure 2 ). Of the 5 patients undergoing primary angioplasty, 3 had eventual stent placement during subsequent procedures. Overall, larger balloons were used in the patients with major systemic venous occlusions ( Table 1 ). Thrombolysis, thrombectomy, or aspiration of thrombus was not performed in any patient.




