Closure of perimembranous ventricular septal defects in patients with Down syndrome, either surgically or by Amplatzer occluders, carries a high risk of complete heart block. We report 5 closures using the transcatheter patch, a wireless bioabsorbable device without any reported heart block to date. The median defect size was 11 mm. Small devices were used in 4 patients and a medium size in 1. The patch was released after 48 hours in 3 patients and immediately in the other 2. Of the 5 patients, 3 were followed up for >5 years and 2 for 1 year. None of these patients had atrioventricular block during their follow-up. In conclusion, the transcatheter patch might be superior in terms of cardiac conduction system protection in patients with Down syndrome after ventricular septal defect closure.
Ventricular septal defect (VSD) closure, either surgical or transcatheter using Amplatzer devices, carries a greater risk of heart block in patients with Down syndrome (DS) compared to the same procedures in genetically normal subjects. However, the number of reported transcatheter procedures is limited. We reported on 5 perimembranous ventricular septal defect (PMVSD) device closures using transcatheter patch (TP, Custom Medical Devices, Athens, Greece) and reviewed the published medical data.
Methods
The international registry of PMVSD closures using the TP was searched for patients with the DS; 5 cases were found. The data of the patients are summarized in Table 1 . The median age and weight were 7 years (range 4 to 11) and 26 kg (range 16 to 36), respectively. All were candidates for VSD closure because of a shunt ratio of ≥1.5:1 and/or pulmonary hypertension. The median VSD size was 11 mm (range 4 to 16).
| Pt. No. | Age (y) | Weight (kg) | VSD Diameter (mm) | Patch Size | Release Method | Complete VSD Closure | Follow-up (y) |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 16 | 12 | Small | Delayed | Yes | >5 |
| 2 | 7 | 20 | 11 | Small | Delayed | Yes | >5 |
| 3 | 7 | 28 | 4 | Small | Immediate | No | 1 |
| 4 | 10 | 26 | 16 | Medium | Delayed | Yes | >5 |
| 5 | 11 | 36 | 10 | Small | Immediate | Yes | 1 |
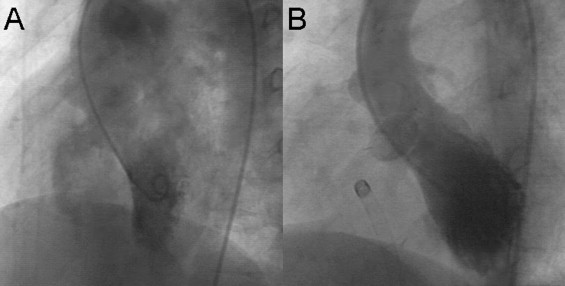
After providing informed consent, the patients were sent to the catheterization laboratory. Of the 5 patients, 3 underwent catheterization under general anesthesia and 2 under deep sedation. The femoral artery and vein were catheterized, and heparin (100 IU/kg) was administered to all patients. Fluoroscopy and transthoracic echocardiography were used to guide the procedures. A left ventriculogram and an aortogram were performed to delineate the VSD anatomy and any existing aortic regurgitation before VSD closure ( Figure 1 ).
The TP is made of porous polyurethane foam, available in 3 sizes (small, medium, and large). The late release method (48 hours) was used for 3 patients, as previously described. In brief, the TP was delivered on the distal balloon of a 2-balloon system. The distal balloon was inflated in the left ventricle and the proximal one in the right ventricle. This secured the patch on the VSD rim for 48 hours, until natural fibrin formation secured the patch position and permitted emptying and removal of the balloons.
Owing to the inconvenience of this method, the immediate release patch was developed. This patch is mounted on a bioabsorbable balloon. The balloon is inflated in the left ventricle and secured by a bioabsorbable thread, which is attached to the patch and is sutured tightly on the groin. The patch is attached naturally to the septum by the time of balloon and thread absorption, normally <6 months after the implantation. This type of TP was implanted in 2 patients.
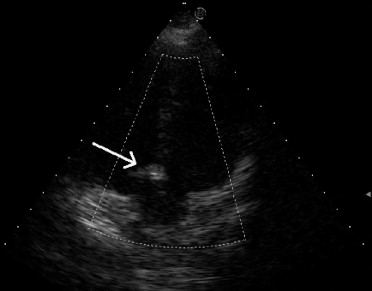
After TP implantation, a left ventriculogram and an aortogram were repeated to document any residual shunt or new aortic regurgitation. The follow-up echocardiograms in patients 3 and 5 showed balloon absorption and patch flattening after 6 months ( Figure 2 ). The follow-up electrocardiograms were obtained 1 month after the procedure, every 3 months during the first year, and annually thereafter.