Chapter 16 Atrial fibrillation (AF) is the most common cardiac arrhythmia, and a major cause of stroke in the elderly.1 An estimated 12 to 16 million Americans will have a diagnosis of AF by 2050.2 The risk of stroke attributable to AF increases with age from 1.5% in the 50- to 59-year-old age group to 23.5% in the 80- to 89-year-old age group.1 The annual incidence of stroke in persons with untreated AF is 4.5%.3 Adjusted odds ratios for mortality in AF are 1.9 in women and 1.5 in men,4 and 1.71 overall.5 Women have strokes later in life, and they are more likely than men to have long-term disability and poor functional status poststroke.6 Oral anticoagulation is recommended for prevention of stroke in AF (class I, level of evidence A)7; however, warfarin treatment requires frequent monitoring to maintain International Normalized Ratio (INR) between 2 and 3.7 In addition, a significant number of drugs commonly used by this population can potentiate warfarin action, including amiodarone, simvastatin, and omeprazole.8 Patients taking warfarin must also adhere to a consistent intake of vitamin K and avoid a number of dietary supplements that can affect coagulation. For example, fish oil potentiates coagulation, whereas ginseng can inhibit warfarin action.8 The direct thrombin inhibitor dabigatran does not require regular monitoring as does warfarin, but it has a similar risk of hemorrhage as warfarin at the higher dose of 150 mg two times daily.9 In addition, dabigatran and the direct Factor Xa inhibitor rivaroxaban are primarily excreted by the kidney; therefore dosages need to be adjusted in persons with chronic kidney disease.10 Alternative therapies are warranted, particularly in patients who are not candidates for anticoagulant therapy or who are at high risk of bleeding. The left atrial appendage (LAA) is a prominent source of thrombi in AF, accounting for 90% of thrombi observed in patients undergoing cardioversion.11 In a small number of patients (3%), the LAA may be a source of focal atria tachycardia.12 As a result, surgical and transcatheter techniques have been explored to reduce the risk of stroke in persons with AF by occluding the LAA. The percentage of complete closure or occlusion is dependent on the modality employed13,14 and the selection of appropriate anatomy. The high variability of LAA anatomy15 does not allow for a “one device fits all” paradigm. Thus several devices are under clinical evaluation or preclinical assessment. History of stroke and/or transient ischemic attack (TIA) was the major predictor of stroke in patients with AF in one analysis (relative risk 2.5; 95% confidence interval [CI] 1.8 to 3.5),16 in addition to diabetes, hypertension, and increasing age. Several scoring systems have been proposed to assess risk of stroke in AF. The most widely used system in clinical practice is the Congestive heart failure, Hypertension, Age >75 years, Diabetes, and Stroke score (CHADS2),17 which combines several risk predictors into a 7-point scale (Box 16–1). Patients with a CHADS2 score of 1 or higher were more likely to have left atrium or LAA thrombus or sludge than those with a CHADS2 score of 0 (3.9% vs. 0%, p < 0.01), and the prevalence of left atrial spontaneous echocardiographic contrast (SEC) increased with increasing CHADS2 score (24% with score of 0; 83% with score of 4 to 6, p < 0.01).18 Obesity is also a risk factor for left atrial and LAA thrombus in AF. Patients with a body mass index (BMI) of 27.0 kg/m2 or higher have a greater prevalence of nonparoxysmal AF (37% vs. 21%, p < .001) and CHADS2 score of 1 or higher (68% vs. 49%, p < .001).19 A higher CHADS2 score was also associated with increased risk of hemorrhagic stroke and death in the RE-LY trial,20 which compared dabigatran and warfarin in AF patients. The American College of Chest Physicians recommends no medical intervention for AF patients with a CHADS2 score of 0.21 The CHADS2 score has been modified to accommodate other risk factors for stroke in AF. Data from the Euro Heart Survey on Atrial Fibrillation were used to develop the 10-point (0 to 9) CHA2DS2-VASc score.22 Determinants of this score are listed in Box 16–1. Low risk is categorized as a CHA2DS2-VASc score of 0, intermediate risk as a score of 1, and high risk as a score 2 or greater. Increased CHA2DS2-VASc score was associated with increased risk of thromboembolic events at 1 year, with 0 events per patient year in the low-risk group, 0.46 events per patient year in the intermediate-risk group, and 1.71 events per patient year in the high-risk group (p < .0001).23 The European Society for Cardiology has recommended that the CHA2DS2-VASc scoring system be used if the CHADS2 score is 0 to 1 or when a more detailed assessment of stroke risk is indicated.24 Patients with a CHA2DS2-VASc score of 0 had no differences in stroke risk in a population-based study, regardless of medication use.25 These scoring systems for stroke risk in AF are highly important because they guide selection of medical treatment or interventions. In addition to comorbid conditions, endothelial dysfunction and platelet activation may play a role in stroke risk in AF. These characteristics are interrelated and may be associated with the obesity, hypertension, and diabetes seen in patients with metabolic syndrome.26 Plasma levels of von Willebrand factor (vWF) were increased in persons with AF relative to those in sinus rhythm, whereas the levels of the vWF protease, a disintegrin and metalloproteinase with a thrombospondin-type 1 motif member 13 (ADAMTS13), were decreased in AF.27 In this study, the vWF/ADAMTS13 ratio was negatively correlated with LAA flow velocity (r = −0.345, p = .002).27 Plasma soluble CD40 ligand, a marker of platelet activation, was correlated with extent of SEC (r = 0.377, p = .02); however, levels of soluble CD40 ligand were not associated with warfarin use.28 Soluble P-selectin and fibrin D-dimer levels, indicative of prolonged platelet activation and coagulability respectively, were increased in 17 patients with AF relative to 34 patients in a sinus rhythm control group.29 Endothelial dysfunction and platelet activation are thus further risk factors for stroke in AF, especially because of their potential for interaction with a prosthetic device in the LAA. The LAA is derived from the embryonic left atrium.30 It is a blind pouch lying on the anterior surface of the heart. Normal anatomic variation in LAA morphology is shown in Figure 16–1. An autopsy study31 showed that 54% of LAAs examined (n = 500) have two lobes, with 23%, 20%, and 3% having three, one, and four lobes, respectively. In addition to multiple lobes, the pectinate muscles create a trabecular structure that may promote thrombus formation.30 In another autopsy study,15 220 casts of the LAA from patients with known medical histories were examined for the course of the principal axis and the number of lobes and finer structures. Ninety-two (42%) casts had an “extremely bent and extremely spiral” course, whereas only 16 casts (7%) had a straight course. The number of lobes and finer structures were significantly correlated with LAA volume and ostium diameter. Hearts from patients who had AF had larger LAA volumes and ostial diameters (both p < .01) and a lower number of lobes (p < .05) than hearts from patients who were in sinus rhythm before death.15 Figure 16–1 Normal anatomic variation of LAA morphology. (Images courtesy of Seattle Science Foundation, Seattle, Wash.) The ostium of the LAA is elliptical in shape,15,31 which may have consequences for the design of occlusion devices.32 The diameter and area of the LAA ostium increase progressively with the severity of AF.33 The pectinate muscles within the LAA may be misread as thrombi on two-dimensional (2D) transesophageal echocardiography (TEE),34 and they may hinder the success of LAA ligation or occlusion. Use of real-time three-dimensional (3D) TEE can help distinguish between pectinate muscles and thrombi35; however, a poor 2D image often predicts an inconclusive real-time 3D TEE result. In addition, 2D TEE underestimates the size of the LAA ostium relative to real-time 3D TEE,33 which can impact the choice of device size. TEE and computed tomography angiography (CTA) are effective modalities to assess left atrial and LAA anatomic and functional features that may increase thrombogenesis in AF.11 SEC on TEE, which may reflect microemboli, is increased in AF.36 The highest amount of SEC is negatively correlated with LAA velocity.36,37 Patients with AF and thrombus have lower LAA velocity than patients without thrombus (no thrombus, 32 ± 21 cm/sec; with thrombus, 9 ± 6 cm/sec; p < .001.36 AF patients with a history of stroke also have larger LAA depth and neck dimensions.38 Increased left atrial volume index (odds ratio [OR] 1.02; p = .018) and lower left ventricular ejection fraction (OR 1.02; p = .05) on TEE measurement can predict LAA thrombus formation. Transthoracic echocardiography (TTE) with measurement of LAA wall velocity (LAWV) was used to assess risk of recurrent stroke in AF patients.39 In this study, patients with TTE-LAWV less than 8.7 cm/sec were more likely to experience recurrent cerebrovascular events (hazard ratio 5.05; 95% CI 2.25 to 11.36).39 The combination of low LAA flow velocity, endothelial dysfunction, platelet activation, and procoagulant state may thus set up an ideal environment for thrombus formation in the LAA. CTA has become an important tool to assess LAA morphology. The image quality may be compromised at times by poor timing of contrast appearance in the LAA. CTA gives an excellent 3D understanding of the orientation of the appendage relative to the pulmonary artery, the number of lobes, and the shape of the appendage as well as the orientation of the appendage—posterior, lateral, or anterior.40 CTA has become essential in the deployment of the SentreHEART LARIAT device (SentreHEART, Palo Alto, Calif.). Wang et al41 have categorized LAA morphology into four categories based on CTA analysis: (1) wind sock (one long, dominant lobe; 46.7% of patients studied); (2) cauliflower (short length with complex internal structure; 29.1%); (3) chicken wing (one prominent bend in LAA; 18.3%); and (4) cactus (dominant central lobe with secondary lobes; 5.9%). The ostium shape and LAA location relative to the left superior pulmonary vein can also be categorized by CTA.41 Patients who have AF with the chicken wing LAA morphology are less likely to have a history of stroke or TIA than the other LAA morphologies (OR 0.21, 95% CI 0.05 to 0.91; p = 0.04).42 The primary drug used for medical management of stroke risk in AF has been warfarin. As discussed, for many patients warfarin is either not tolerated or interferes with other medications or lifestyle. Major gastrointestinal bleeding and hemorrhagic stroke are principal adverse events with warfarin or the newer oral anticoagulants. Two bleeding risk scales have been validated for patients on oral anticoagulant therapy, with the acronyms HEMORR2HAGES43 and HAS-BLED.44 The constituents of these scales are listed in Box 16–2. The HAS-BLED scale outperformed the HEMORR2HAGES scale in the SPORTIF trial of the direct thrombin inhibitor ximelagatran and warfarin in showing a stepwise increase in rates of major bleeding with higher scores.45 Patients with a high risk of bleeding are natural choices for LAA occlusion, with the caveat that these devices are noninferior to warfarin regarding stroke prevention.46 Although anticoagulant therapy is recommended to reduce stroke risk in AF,7 alternative strategies are needed for patients who have contraindications to use of anticoagulants. Hence, exclusion of the LAA has emerged as a treatment option. Three approaches have been developed to exclude the LAA. Open surgery for LAA exclusion or removal has been relegated at this time to combined coronary artery bypass graft (CABG) or valve surgery. Epicardial LAA occlusion via ligation has been used, as well as a hybrid epicardial/endovascular approach. Finally, several endovascular LAA occlusion devices are currently under evaluation (Figure 16–2). The rates of complete occlusion are highly variable47,48; additionally, periprocedural adverse event rates may be dependent on operator experience.49 Figure 16–2 LAA occlusion devices discussed in this review. The Left Atrial Appendage Occlusion Study (LAAOS)13 was the first randomized, single-center study of surgical LAA occlusion in patients undergoing concurrent CABG with staples or sutures versus a control group. Seventy-seven patients were randomized either to undergo occlusion (n = 52) or the control group (no occlusion; n = 25); only 11 (14%) had a history of AF. TEE was used to test degree of occlusion at 8 postoperative weeks in 44 of 52 (85%) patients. Occlusion was successful in 29 (66%) patients, more so when stapling was employed (72%) rather than sutures (45%, p = .14). Two patients, both in the occlusion group, had thromboembolic events during hospitalization. One patient with AF, patent foramen ovale, and bilateral carotid stenosis had an intraoperative stroke; the other patient had a TIA on the third postoperative day. Importantly, after a follow-up period of 13 ± 7 months, no additional stroke events were reported in either occlusion or control groups.13 In one retrospective series,50 six females with AF underwent LAA occlusion concurrently with mitral or aortic valve surgery. The LAA was occluded with running sutures, and no TEE was performed intraoperatively. At follow-up TEE (23 to 159 postoperative days), only one of the patients had complete closure of the LAA ostium. All of the other five patients had postoperative SEC within the LAA, which was more serious than it was before the occlusion in two patients. One patient with incomplete occlusion of the LAA had a stroke 4 weeks postoperatively despite having an INR of 5.9.50 The authors concluded that intraoperative TEE was necessary to assure complete closure of the LAA and reduce risk of postoperative stroke. The primary issue limiting the use of surgical LAA occlusion is the variability of complete closure, ranging from 17% to 93%.51 It should be noted that there is a difference between the anatomical ostium and the TEE-defined ostium of the LAA31 that may adversely affect occlusion regardless of the approach. Inadequate TEE visualization of the ostium during surgery may result in incomplete closure of the LAA, which allows continued formation of thrombus within the structure. A major concern is whether incomplete occlusion is worse than no occlusion, given that reduced blood flow velocity in the LAA may enable more thrombus formation than in the fully patent situation. Kanderian et al52 performed a retrospective analysis of 137 patients who underwent surgical LAA occlusion and had TEE evaluation 8 ± 12 months after surgery. Excision resulted in a 73% successful closure rate, compared with 23% for suture exclusion and 0% for stapler exclusion (p < .001). Thrombus was visualized within the LAA in 28 of 68 (41%) patients who had unsuccessful closure after suture or stapler exclusion; however, 11% of patients who had successful closure versus 15% of patients who had unsuccessful closure suffered a stroke or TIA after surgery (p = .61). Based on these findings, the authors identified four outcomes of surgical LAA closure: (1) successful closure; (2) patent LAA, defined as persistent communication of the LAA with left atrium as a result of dehiscence; (3) excluded LAA with persistent flow, defined as color-flow jet between LAA and left atrium despite appearance of closure; and (4) remnant LAA with pouch greater than 1 cm in length. The authors concluded that anticoagulation therapy should not be discontinued until successful closure is confirmed by TEE evaluation.52 Epicardial methods for LAA occlusion are in development, and some devices are being used clinically. Procedures can be performed concurrent with open surgery or via endovascular techniques. The AtriCure AtriClip system (Cincinnati, OH) (Figure 16–3) was used in 34 patients with AF who were undergoing concurrent CABG or valve replacement. The LAA was successfully occluded in all patients after 3 months, as assessed by TEE.53 Epicardial exclusion of the LAA may have other beneficial effects in AF. In a small case series (N=10) utilizing the AtriClip system54 in concert with pulmonary vein isolation and off-pump coronary artery bypass, 100% of patients achieved acute electrical isolation of the LAA. The Aegis system (Aegis Medical Innovations, Inc., Vancouver, Canada), a percutaneous device, consists of a “grabber” with integrated electrodes to identify the LAA under fluoroscopic guidance, and a ligator (hollow suture) to cinch the LAA at the base. Ligation of the LAA was successful in five out of six dogs in a preclinical study.55 Necropsy after 2 to 3 months revealed an atretic remnant LAA in the animals. A silicone band delivered via a percutaneous system (Cardioblate; Medtronic, Minneapolis, Minn.) resulted in complete occlusion of the LAA in 15 out of 15 dogs studied.56 A combination epicardial/endovascular procedure, consisting of the LARIAT suture delivery device and magnetic-tipped guidewires with a balloon-tipped endovascular catheter, was initially tested in 10 dogs.57 Balloon inflation to confirm the position of the LAA resulted in complete closure in five out of five dogs, whereas a residual proximal LAA was seen in three of four dogs without balloon inflation (p = .05). A larger study involving 26 dogs demonstrated complete LAA closure in 100% of the dogs.58 A subset of 10 dogs was studied for 7 days (n = 3), 1 month (n = 3), and 3 months (n = 4). Histological examination demonstrated an atretic LAA with complete endothelialization at the closure site by day 7.58 A small (n = 13) nonrandomized study59 has been completed on LAA ligation using the SentreHEART system. The majority (11/13, 85%) of these patients underwent concurrent catheter ablation, and the remaining two patients (15%) underwent concurrent mitral valve replacement. Ten of the 11 patients undergoing catheter ablation and both of those with mitral valve replacement achieved successful ligation of the LAA. Follow-up TEE performed 60 days after ligation in six patients revealed complete closure in four (67%); the remaining two patients had color-flow jet less than 2 mm on color flow Doppler.59 A larger, single-center observational study consisting of 119 patients assessing the efficacy of LAA closure and procedural safety with the SentreHEART system has been reported.60 Sixteen patients were excluded by computed tomography because of LAA orientation behind the pulmonary artery or LAA diameter larger than 40 mm. Fourteen patients were excluded as a result of thrombus identified during TEE or adhesions. Of the 85 patients undergoing LAA ligation with the LARIAT suture delivery device, 96% of the LAA ligations resulted in complete closure, with the remaining patient having a leak less than 2 mm as seen by Doppler echocardiography at the 1-year follow up examination. There were no device-related complications. Three access-related complications occurred in three patients. The adverse events included chest pain (24%), pericarditis (2.4%), late pericardial effusion (1.2%), and late thrombus formation (1.2%).60 LAA ligation with the LARIAT suture device appears to be effective at excluding the LAA with acceptably low access rate of complications. However, none of the epicardial procedures discussed have been assessed for long-term reduction of stroke risk in humans with AF.
Transcatheter Left Atrial Appendage Occlusion
16.1 Risk of Ischemic Stroke in Atrial Fibrillation
16.2 The Left Atrial Appendage
Anatomic Considerations
Diagnostic Considerations
Indications for Left Atrial Appendage Occlusion and Patient Selection
16.3 Left Atrial Appendage Exclusion Modalities
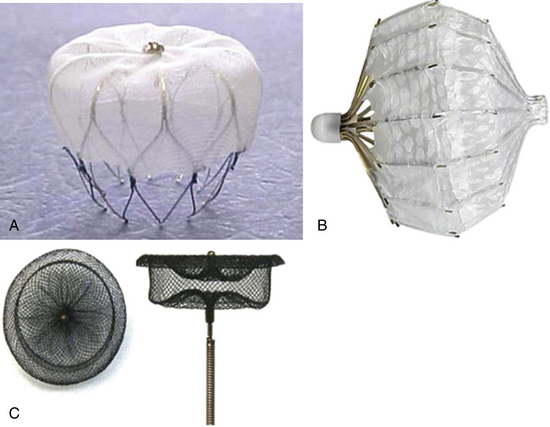
A, WATCHMAN device. B, PLAATO device. C, Amplatzer cardiac plug. (Adapted from Sievert H, Lesh MD, Trepels T, et al: Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience, Circulation 105(16):1887-1889, 2002.)
Open Surgical Approaches
Epicardial Left Atrial Appendage Occlusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine