20 Transcatheter Closure of Atrial Septal Defects and Patent Foramen Ovale
Secundum ASD
Clinical Presentation
Symptoms may include the following:
• Reduced exercise tolerance or fatigue
• Palpitations (due to supraventricular arrhythmias, frequent atrial fibrillation/atrial flutter in older age), or syncope for sick sinus syndrome
• Atypical chest pain (right ventricular ischemia)
• Frequent respiratory tract infections
• Signs of right-heart failure
• Paradoxical embolism from peripheral venous or pelvic vein thrombosis, atrial arrhythmias, unfiltered intravenous infusion, or indwelling venous catheters
Echocardiography
Table 20-1 summarizes the key points regarding TEE evaluation of intracardiac anatomy.
Table 20-1 Transesophageal Echocardiogram Features to Consider for Atrial Septal Defect Closure
Contraindications for Percutaneous Closure of Secundum ASD
An absolute contraindication for percutaneous ASD closure is the presence of severe and irreversible PAH, with no evidence of a left-to-right shunt. See Table 20-2 for a summary of the indications and contraindications to ASD closure.
Table 20-2 Indications and Contraindications to Atrial Septal Defect Closure
ASD Closure Devices
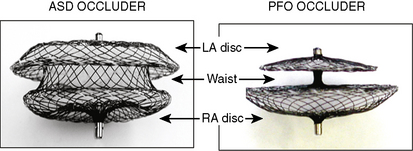
Amplatzer Septal Occluder (ASO)
The Amplatzer septal occluder (ASO) is a self-expandable double disc device made of nitinol (55% nickel, 45% titanium) wire mesh. The ASO is tightly woven into two flat discs (Fig. 20-1). There is a 3- to 4-mm connecting waist between the two discs, corresponding to the thickness of the atrial septum. Nitinol has super elastic properties, with shape memory. This allows the device to be stretched into an almost linear configuration and placed inside a small sheath for delivery and then return to its original configuration within the heart when not constrained by the sheath. The device size is determined by the diameter of its waist and is constructed in various sizes ranging from 4 to 40 mm (1-mm increments up to 20 mm; 2-mm increments up to the largest device currently available, 40 mm; the 40-mm size is not available in the United States). The two flat discs extend radially beyond the central waist to provide secure anchorage.
Amplatzer Delivery System
For device deployment, we recommend using appropriately sized sheaths for the device as summarized in Table 20-3. The delivery system is supplied sterilized and separate from the device. It contains all the equipment needed to facilitate device deployment. It consists of the following:
1. Delivery sheath of specified French size and length, and appropriate dilator
2. Loading device, used to collapse the device and introduce it into the delivery sheath
3. Delivery cable (internal diameter [ID] 0.081 inch): the device is screwed onto its distal end allowing for loading for loading, placement, and retrieval of the device
4. Plastic Pin-vice: this facilitates unscrewing of the delivery cable from the device during device deployment
5. Tuohy Borst valve adapter with a side arm for the sheath, to act as a one-way stop-bleed valve
Table 20-3 Sheath Delivery System Sizing for Atrial Septal Defect Devices
Optional but Recommended Equipment
Amplatzer Super Stiff Guidewire
The 0.035-inch Amplatzer super stiff exchange guidewire is used to advance the delivery sheath and dilator into the left upper pulmonary vein. Table 20-4 summarizes all the necessary materials for ASD closure.
Table 20-4 Materials/Equipment Required for Transcatheter ASD Closure Procedures
| Item | Size | Cost each (US$) |
|---|---|---|
| Amplatzer Septal Occluder | 4–40 mm | 5500 |
| Amplatzer PFO Occluder | 18, 25, 35 mm | 5000 |
| Amplatzer Delivery System | 7–12F | 580 |
| Amplatzer Super Stiff exchange | 0.035-inch, 100 cm length guidewire | 45 |
| Multipurpose catheter | 6–7F | 10 |
| Amplatzer Sizing Balloon | 24, 34 mm | 265 |
| Amplatzer Rescue System | 9 F, 12F | 580 |
PFO, patent foremen ovale.
Step-by-Step Technique: Transcatheter Device Closure of Secundum ASD
Materials and Equipment
1. Single- or bi-plane cardiac catheterization laboratory
3. Full range of device sizes, delivery and exchange (rescue) systems, sizing balloons
4. A multipurpose catheter to engage the defect and the left upper pulmonary vein
5. Extra-stiff exchange length wire, for example, a 0.035-inch Amplatzer super stiff exchange length guidewire with a 1-cm floppy tip, but any extra-stiff J-tipped wire may be used.
Personnel
Method
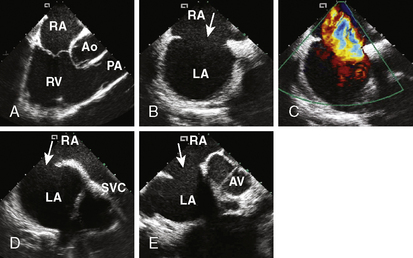
4. Echocardiographic assessment of the secundum ASD is performed simultaneously using either TEE or ICE. Figure 20-2 demonstrates full assessment of the defect by ICE.
The important ASD rims to look for are:
• Superior/SVC rim—best achieved using the bicaval view
• Superior posterior/right upper pulmonary vein rim
• Anterior superior/aortic rim—the least important rim; often, patients lack it
• Inferior/IVC and coronary sinus rim—an important rim to have
• Posterior rim—seen best in the short-axis view at the aortic valve level
6. Perform a right upper pulmonary vein angiogram (Fig. 20-3A) in the hepatoclavicular projection (35-degree left anterior oblique/35-degree cranial). This delineates the anatomy, shape, and length of the septum. This may come in handy when the device is deployed but not released—the operator can position the imaging tube in the same view of the angiogram and compare the position of the device with that obtained during the deployment (Fig. 20-3B, C).