Transaortic Endarterectomy
Margaret C. Tracci
Kenneth J. Cherry
Gilbert R. Upchurch Jr.
Transaortic endarterectomy for the treatment of aortoiliac occlusive disease, when performed by a skilled surgeon in carefully selected patients, offers excellent long-term durability and avoids many of the common failure modes of the open and endovascular techniques by which it has largely been supplanted.
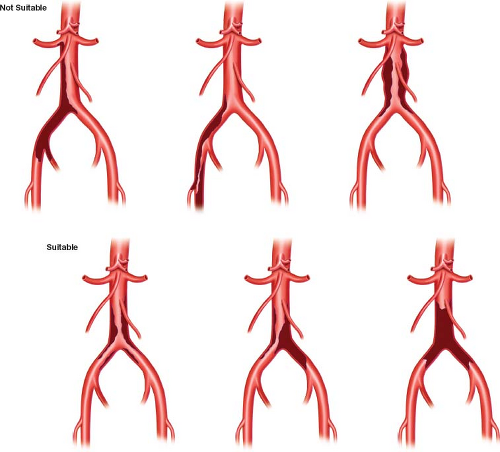
Once established, the technique of endarterectomy was initially applied very broadly. Subsequent accumulation of experience with complementary techniques of endarterectomy and bypass led to more limited application to disease restricted to the distal infrarenal aorta and common iliac arteries. Darling and Brewster very elegantly compared 11-year results for aortoiliac and aortoiliofemoral endarterectomy to aortoiliac and aortofemoral bypass grafting performed over the same time period and demonstrated, as confirmed by others, that the patency and complication rates favored applying the technique of endarterectomy to disease of the terminal aorta and one or both common iliac arteries, rather than pursuing lesions down the external iliac. Disease at this location has been characterized as type I (involving the infrarenal aorta, common iliac, and internal iliac arteries) versus type II disease (extending through the external iliac and common femoral bifurcation). The latter has been described as more common (4:1) and predominant in males (5:1 male vs. 2:1 female) (Fig. 20.1). The younger patient typical of type I disease may be better suited for the longer operative duration and more substantial blood loss associated with endarterectomy, and may also benefit from the durability of this technique of reconstruction.
Patients in whom synthetic graft reconstruction is inadvisable, either due to the presence of an infected graft either with or without complicating aortoduodenal fistula, or other infectious threat, may also be considered for endarterectomy. The presence of erectile dysfunction (ED) in the setting of severe aortoiliac occlusive disease also favors endarterectomy, as several groups have reported that 30% to 50% of patients treated in this manner may experience improvement of ED, an obvious advantage of reconstructing the affected hypogastric and restoring antegrade flow, as is often not accomplished effectively by aortofemoral bypass grafting. These circumstances may reasonably lead a surgeon to contemplate either treating more extensive disease than the type I distribution described above or to extend the technique to older patients than might otherwise be selected.
Evidence of aneurysmal dilation of the aorta or iliac arteries is perhaps the only absolute contraindication to this technique, as it would tend to predispose the patient to further aneurysm degeneration of the thinned wall over time. Circumferential calcification of the abdominal aorta that precludes effective clamp placement is another technical barrier to both endarterectomy and bypass operations. Relative contraindications based on anatomic considerations include extensive type II disease, which requires a more technically demanding endarterectomy of the external iliac and even femoral arteries, and is associated in many reports with a higher rate of complications and early and late failures or extremely tortuous iliac vessels. Some would consider very small iliac vessels to be a relative contraindication. However, except where the external iliac
artery has regressed to a mere cord, the vessels tend to enlarge following endarterectomy and the narrowing of the vessels associated with open endarterectomy and primary closure can be addressed either through use of a semi-closed technique or by patch closure. The age and physical condition of the patient constitute the other major considerations in selecting a method of open surgical or endovascular reconstruction.
artery has regressed to a mere cord, the vessels tend to enlarge following endarterectomy and the narrowing of the vessels associated with open endarterectomy and primary closure can be addressed either through use of a semi-closed technique or by patch closure. The age and physical condition of the patient constitute the other major considerations in selecting a method of open surgical or endovascular reconstruction.
A thorough history should be obtained, both to define the extent and nature of symptoms referable to the patient’s aortoiliac occlusive disease and to identify important comorbidities. As the incidence of comorbid coronary and cerebrovascular disease in this patient population is significant, careful attention to these elements of the history is essential. As per institutional or practice protocol, the application of risk assessment guidelines, such as the Revised Cardiac Risk Index (RCRI) or Fleisher–Eagle criteria, taking into account: (1) Patient-specific clinical variables, with a focus on ischemic, coronary, or valvular heart disease, cerebrovascular disease, diabetes, and renal function; (2) exercise capacity; and (3) surgery-specific risk, may guide decision making regarding the necessity of further preoperative testing. The authors typically utilize duplex ultrasound to evaluate for hemodynamically significant carotid disease and have a low threshold for noninvasive cardiac testing comprising both physiologic or pharmacologic stress testing and radionuclide myocardial perfusion imaging. This is based on the relatively high-risk nature of aortic surgery, the prevalence of other risk factors for cardiovascular events in this population, and the unreliability of historical assessment of functional capacity based on the limiting effects of the disease being treated. This is consistent with the excess in postprocedure events observed in patients undergoing aortic surgery relative to other noncardiac surgery patients in validation of the RCRI. Laboratory assessment of hematocrit and renal function is also routinely performed. Appropriate risk-factor modification should be instituted, including, in most patients, initiation or maintenance of aspirin, a statin, and beta blocker. Blood pressure and blood glucose levels should be well managed before embarking on operation. Smoking cessation is to be strongly encouraged. While there is benefit to continuing aspirin therapy up to surgery and through the perioperative period, we are less sanguine about the continuation of thienopyridines, warfarin, and other antiplatelet or anticoagulant medications. Patients with valvular heart disease may require bridging therapy with unfractionated or low molecular weight heparin.
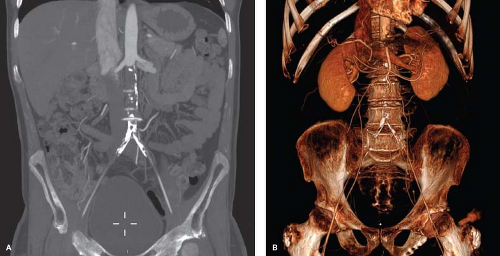
Technical planning is largely based on the availability of excellent preoperative imaging. It is essential that the surgeon develops a thorough familiarity with the patient’s anatomy and extent of occlusive disease and has a clear plan for the procedure that accounts for the likelihood of encountering anatomic issues that may require a change in technique or plan, such as significant aortic calcification or distal disease more severe than initially thought. The historical gold standard was digital subtraction angiography. Angiography continues to provide excellent visualization of vessel lumen. More recently, noninvasive testing has largely supplanted catheter-based imaging for preoperative planning. Magnetic resonance angiography with gadolinium-based contrast medium provides excellent anatomic imaging with fine soft tissue detail. It can tend to overestimate the degree of stenosis and is not effective in visualization of calcium, which may, in this context, affect both clamp placement and endarterectomy. Where possible, the authors prefer CT angiography, which provides anatomic detail, assessment of calcium, and better resolution than that currently afforded by magnetic resonance techniques.
It is important that the patient maintains adequate hydration prior to surgery. For this reason, we do not advocate aggressive bowel preparation that would tend to predispose a patient to dehydration. With regard to prevention of wound complications, the patients are asked to shower with a chlorhexidine solution. Hair removal is performed carefully, using clippers, by the operating room staff. Prophylactic antibiotics are administered within an hour of incision, typically a first-generation cephalosporin or appropriate alternative for allergic patients, and subcutaneous unfractionated or low
molecular weight heparin is given preoperatively, as well, for the prevention of deep venous thrombosis.
molecular weight heparin is given preoperatively, as well, for the prevention of deep venous thrombosis.
While use of a Swan–Ganz catheter is no longer considered mandatory, the authors continue to advocate continuous blood pressure monitoring via an arterial catheter and placement of central venous access. Epidural anesthesia is also favored for improved pain control in the postoperative period. Use of an intraoperative cell salvage machine is recommended as well to minimize the necessity of donor blood transfusions. A nasogastric tube and indwelling urinary catheter are typically placed and remain through at least the initial postoperative period.
Positioning
While both retroperitoneal and transperitoneal techniques have been described, the authors generally favor a midline transperitoneal approach, with the patient in the supine position. The arms may be either tucked or extended, based on the surgeon’s preference. In either case, careful attention to padding of bony prominences and pressure points and avoidance of hyperextension of the brachial plexus, particularly with the arms extended, are essential. The patient is secured to the table with a well-padded safety strap. If warming blankets are utilized, restricting their application to the upper body minimizes the risk of thermal injury associated with active warming of a nonperfused lower extremity. The surgeon stands to the right side of the patient while the assistant is positioned to the left. The lower chest, abdomen, both groins and proximal thighs are prepped with a chlorhexidine solution and then draped with sterile towels and sheets. The authors typically utilize an antimicrobial adhesive drape on the dried and prepped skin after towels and sheets are applied.
Technique
Open and semi-closed techniques of endarterectomy will be presented, including modifications to address clinical situations, such as narrow vessels, difficult distal endpoints, and extensive disease of the external iliac and femoral arteries.
Dissection
The surgeon makes a generous midline laparotomy incision. The incision is carried through the subcutaneous tissue and the fascia opened carefully at the linea alba. The small intestine is gently reflected to the patient’s right side and the retroperitoneum incised longitudinally over the infrarenal aorta, permitting mobilization of the duodenum. A self-retaining retractor is placed to aid in exposure. The authors favor the Omni-Tract (Integra LifeSciences, Plainsboro, NJ). Other self-retaining retractors such as the Thompson Retractor System (Thompson Surgical Instruments, Inc. Traverse City, MI) are also suitable. The authors have found the standard-sized Bookwalter (Codman & Shurtleff, Inc, Raynham, MA) retractor widely used in general surgery to be inadequate for larger patients, although the Codman Company has developed wishbone variations and larger or segmented rings to address the large or obese patient. A damp, radiopaque surgical towel or a large laparotomy pad is used with either a fence or slotted malleable retractor to hold the bowel aside. The splanchnic and renal vein retractors are critical for providing adequate exposure both at the proximal level and in the pelvis. The left renal vein is identified and can be gently mobilized to facilitate clamp placement in an appropriately soft segment of aorta. Management of proximal disease involving the paravisceral segment is discussed in depth in Chapter 11. A renal vein retractor gently applied should expose this vessel up to the level of the renal arteries. Ligation and division of renal vein tributaries is not typically necessary to facilitate this exposure.
Exposure is then continued distally along the right-anterior surface of the aorta, with care taken to avoid and preserve the inferior mesenteric artery. The common iliac arteries may be exposed anteriorly to the level of the iliac bifurcation in the avascular plane of Leriche. This avoids dissection of the adventitia at the level of the aortic bifurcation and proximal iliac arteries which can be associated with disruption of the pelvic sympathetic nerve plexus. This is commonly located below the inferior mesenteric artery origin and courses to the pelvis over the aortic bifurcation, just proximal portion of the left common iliac artery (Fig. 20.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree