Fig. 7.1
Robotic AH-TECAB: port placement: a robotic 3-D camera trocar (C) is placed in the fourth ICS, near the anterior axillary line. The right and left robotic arm trocars are placed in the third (R) and seventh (L) ICS in the mid-clavicular line. An assistant port (A) is created in the fourth ICS, just left lateral from the sternum. A fourth arm EndoWrist™ suction coronary stabilizer (S) is passed through a subcostal peri-xiphoid port for difficult coronary artery exposure
Cardiopulmonary Perfusion and Myocardial Protection
The anterior femoral artery and vein surface should be exposed through a transverse left groin incision without vessel isolation. A 4-0 polypropylene purse-string suture is placed in each vessel near the inguinal ligament. Following administration of intravenous heparin (10,000 units) an 8-French distal leg perfusion cannula is placed using the modified Seldinger technique to avoid leg ischemia, which can be associated with femoral arterial cannulation. Next, a remote access perfusion cannula with a side arm (21 F or 23 F) is introduced into the left femoral artery and the femoral vein is cannulated with a 25-F Quickdraw™ venous cannula (Edwards Lifesciences, Irvine, CA.). An IntraClude™ occlusion balloon catheter (Edwards Lifesciences, Irvine, CA.) is then inserted through the arterial cannula side arm, over a guide wire, and passed into the aortic root. After full systemic heparinization, cardiopulmonary bypass (CPB) is established slowly, while watching for high arterial perfusion pressures that may indicate an early retrograde aortic dissection. Prior to inflating the occluding endoballoon, we induce cardiac asystole with an adenosine injection through the distal port of the clamp. The inflated balloon should be positioned above the aortic sinotubular junction, but well below the innominate artery origin. It is imperative that all cannulation steps and balloon clamp placement be guided by high-quality echocardiography. Antegrade cold blood cardioplegia should be administered every 15 min through the distal catheter tip.
Arrested Heart: Totally Endoscopic Coronary Bypass (AH-TECAB)
Using the robotic endoscope, positioned in the 30° up position, the left internal thoracic artery (LITA) is localized and both the endothoracic fascia and transverse thoracic muscle are divided to expose the vessel. Although in the open chest many prefer internal thoracic artery pedicle harvesting, we skeletonize the vessel. This method enhances conduit visualization during harvesting, provides a longer graft that is suited better for constructing Y- and sequential grafts, and simplifies endoscopic transit-time Doppler flow assessments. Using the robotic spatula cautery, the LITA is teased from loose areolar tissue that connects the artery to associated venae comitantes along the entire length. The electrocautery (set at 15 W) is used to divide most side branches, reserving endoscopic clips for larger ones (Fig. 7.2). During LITA harvesting, intravenous heparin is administered to achieve an activated clotting time of 450 s or greater. The LITA is clipped, divided, and allowed to auto-dilate. For the assistant’s working access, a 5-mm parasternal instrument port is inserted either in the third or fourth intercostal space, and the endoscope is reversed to the 30° down position.
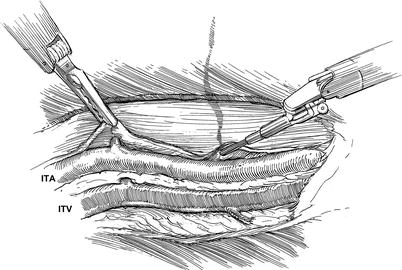

Fig. 7.2
Robotic AH-TECAB: Internal Thoracic Artery (ITA) exposure and skeletonization: to expose the ITA, the endo-thoracic fascia and trans-thoracic muscle layers are divided with a right arm spatula electrocautery. The ITA then is dissected free of the accompanying internal thoracic veins (ITV). Here, a left arm fine tissue robotic forcep is retracting the endothoracic fascia to expose a side branch, which either can be cauterized if small or clipped
The left intra-thoracic space is limited by heart volume and geometry. This limitation creates additional challenges compared to robotic procedures performed from the right hemi-thorax. To perform an AH-TECAB with facility, extensive instrument excursions are required compared to either robotic mitral valve repairs or atrial septal defect closures. It is imperative to place ports and maintain robotic instruments under visual control at all times to avoid cardiac or other intra-thoracic injuries. The subcostal port is inserted just under costal margin to avoid entering the peritoneum and injuring the abdominal organs. Accumulated blood should be aspirated intermittently with a tracheal sucker to prevent intra-thoracic space compromise. To avoid losing “bulldog clips”, silastic loops, needles and other devices in the thorax, all materials should be “parked” in one easy access location and removed while the heart is arrested.
Pericardial fat pad excision (cautery set at 30 W) increases the working-space and enhances coronary target visualization. The fat pad should be brought laterally, starting at the cephalic margin and continuing to the diaphragm, working laterally. Next, the pericardium is incised near the pulmonary artery with exposure continuing toward the sternal border inferiorly to the diaphragm, and then laterally along the diaphragm (Fig. 7.3). The incised pericardium falls postero-laterally by the weight of the fat pad, exposing the heart. The left atrial appendage and phrenic nerve must be identified to avoid inadvertent injury. A bulldog clamp is positioned along the proximal LITA and the distal graft is spatulated and blood flow assessed. Next, the left anterior descending coronary artery (LAD) and diagonal vessels should be identified. Following cardiac arrest, the LAD should be exposed with robotic Potts scissors by opening the overlying epicardium. A robotic lancet blade is used to open the LAD while cardioplegia is administered to distend it, helping to avoid back wall injury (Fig. 7.4). The LITA then is anastomosed to the LAD robotically, using a 7/0 Pronova™ running suture (Johnson and Johnson, Inc., New Brunswick, NJ) (Fig. 7.5). We place the first stitch “inside out” on the LAD and park the needle in the inferior myocardium. Continuing with the second suture arm, we begin sewing “inside out” on the LITA graft and “outside in” along the anastomotic back wall, proceeding from the toe toward the heel. After three stitches, the LITA graft is parachuted onto the LAD surface with sutures continuing around the heel The anastomosis is completed along the anterior wall with the first suture, working from the toe “outside in” on the LITA and “inside out” on the coronary artery. After the suture is tied, hemostasis should be confirmed and graft patency assessed with transit-time flow Doppler. Thereafter, the aortic occlusion balloon is deflated. After CPB weaning, perfusion cannulas are removed and protamine is administered. Using the endoscope, instrument port sites should be inspected for bleeding. A laparoscopic suction-irrigator is used to aspirate any remaining intra-thoracic blood, and a chest tube is inserted through the camera port.
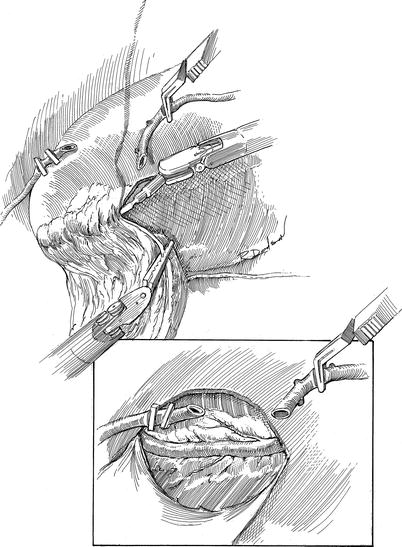
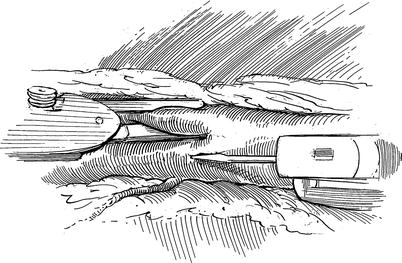

Fig. 7.3
Robotic AH-TECAB: pericardial and LAD coronary exposure: the large pericardial fat pad is removed with the robotic cautery spatula, starting near the sternal margin and dropping it toward the posterior thorax. This exposes the pericardium, which is opened longitudinally to identify the LAD (Inset). After heparinization, the internal thoracic artery has is divided and spatulated in preparation for grafting

Fig. 7.4




Robotic AH-TECAB: coronary arteriotomy: using the miniature scalpel in the right robotic arm, the left anterior descending coronary artery arteriotomy is being made. Silastic band occlusion is necessary only if residual blood cardioplegia continues to obstruct the operative field
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


