In patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention (pPCI), early reperfusion is believed to improve left ventricular systolic function and reduce mortality; however, long-term (>1 year) data are sparse. In the DANish Trial in Acute Myocardial Infarction-2 (DANAMI-2) study, 686 patients with ST-segment elevation myocardial infarction were treated with pPCI. Long-term mortality was obtained during 3 years of follow-up. We classified the patients according to the symptom-to-balloon time (<3, 3 to 5, and ≥5 hours). The groups were compared using a Cox proportional hazards regression model adjusted for confounding factors. The left ventricular systolic ejection fraction was estimated by echocardiography before discharge. Coronary flow was evaluated using the Thrombolysis In Myocardial Infarction score. Mortality did not differ between the 2 earliest symptom-to-balloon groups, and they were therefore combined into 1 group in the analysis of survival. Mortality was significantly increased for patients with a symptom-to-balloon time ≥5 hours (hazard ratio 2.36, 95% confidence interval 1.51 to 3.67, p <0.001), a difference that remained significant after controlling for confounding factors (adjusted hazard ratio 2.44, 95% confidence interval 1.31 to 4.54, p = 0.007). The symptom-to-balloon time was inversely associated with a left ventricular systolic ejection fraction of ≤40% (19.7% vs 22.8% vs 33.1%, p = 0.036), with the latter a major predictor of 3-year mortality in this cohort (hazard ratio 6.02, 95% confidence interval 3.68 to 9.85, p <0.001). A shorter symptom-to-balloon time was associated with greater rates of Thrombolysis In Myocardial Infarction 3 flow after pPCI (86.5% vs 80.9% vs 75.7%, p = 0.002). In conclusion, a shorter symptom-to-balloon time was associated with improved coronary flow, an increased likelihood of subsequent left ventricular systolic ejection fraction >40%, and greater 3-year survival in patients with ST-segment elevation myocardial infarction treated with pPCI.
In the treatment of ST-segment elevation myocardial infarction (STEMI), it has been believed that timely restoration of the coronary artery blood flow using primary percutaneous coronary intervention (pPCI) salvages the myocardium and decreases mortality. However, the currently available data for both these issues have reached conflicting results, and most studies have had a follow-up of <1 year. Moreover, most studies assessing the effect of time delays on mortality in patients with STEMI who were treated with pPCI did not include patients transferred from referral hospitals. The present study assessed the effect of the symptom-to-balloon time on 3-year mortality, left ventricular ejection fraction (LVEF) assessed by echocardiography, and coronary flow after pPCI.
Methods
The DANish Trial in Acute Myocardial Infarction-2 (DANAMI-2) study has been reported in detail in 2 previous publications. In brief, we randomly assigned patients with STEMI to pPCI with stent implantation or front-loaded fibrinolytic therapy with a tissue plasminogen activator (alteplase) and followed them prospectively for 3 years. The patients were enrolled at 24 referral hospitals without angioplasty facilities and at 5 angioplasty centers with on-site surgical backup. The primary outcome of the DANAMI-2 study was a composite of total mortality, clinical reinfarction, and disabling stroke after 30 days of follow-up. Furthermore, we have reported on the composite outcome after 3 years of follow-up. A total of 1,572 patients were enrolled from December 1997 to October 2001. The criteria for inclusion were age of ≥18 years, the presence of symptoms for ≥30 minutes but <12 hours, and ST-segment elevation of ≥0.2 mV in at least 2 contiguous leads. The criteria for exclusion were a contraindication to fibrinolysis, left bundle branch block, acute myocardial infarction or fibrinolytic treatment within the previous 30 days, pulseless femoral arteries, previous coronary bypass surgery, chronic renal failure, diabetes treated with metformin, and noncardiac disease associated with a life expectancy of <12 months. The patients who were judged to be unsuitable for transportation because of cardiogenic shock or severe heart failure (a sustained systolic blood pressure of ≤65 mm Hg), persistent life-threatening arrhythmias, or a need for mechanical ventilation were excluded from randomization. The study complied with the Declaration of Helsinki and was approved by the National Ethics Committee of Denmark. All patients provided written informed consent.
Primary angioplasty was performed on a 24-hour basis, 7 days each week. The patients were treated with aspirin, β blockers, and unfractionated heparin. Stenting of the culprit lesion was attempted in all patients, unless the vessel had a diameter of <2.0 mm. Only the culprit artery was treated at the index angioplasty. Ticlopidine (500 mg) or clopidogrel (75 mg) was given daily for 1 month after stent implantation. Platelet glycoprotein IIb/IIIa receptor blockers were administered at the discretion of the pPCI operator.
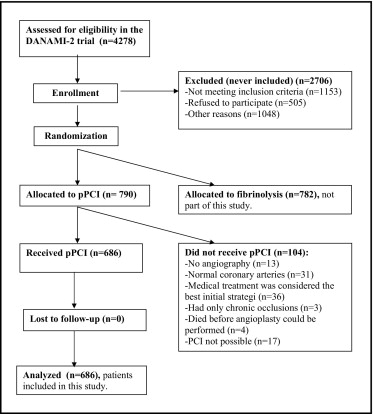
In the present substudy, we included all DANAMI-2 patients assigned to primary angioplasty and in whom angioplasty was actually performed ( Figure 1 ). Our primary objective was to analyze the effect of the symptom-to-balloon interval on 3-year mortality, predischarge LVEF and coronary flow rate (Thrombolysis In Myocardial Infarction [TIMI] flow). Second, we considered the door-to-balloon time as a predictor of 3-year mortality, LVEF, and TIMI flow. We divided the patients into 3 groups with a symptom-to-balloon interval of <3 hours (early), 3 to 5 hours (intermediate), and >5 hours (late), and second, with respect to the door-to-balloon time (<90, 90 to 105, and ≥105 minutes). We decided, a priori, to have the groups reflect tertiles adjusted to the closest hour for the symptom-to-balloon time and to the closest 15 minutes for the door-to-balloon time. Follow-up was performed as per the protocol at 30 days and 1, 2, and 3 years after the index infarction at the hospital at which the patients had been randomized. A 100% follow-up rate was obtained. The symptom onset-to-balloon time was defined as the interval from symptom onset to the first balloon dilation. The door-to-balloon time was defined as the interval from arrival at the first hospital (in most cases, a noninvasive referral hospital) to the first balloon dilation. Echocardiography was performed before discharge, and we recommended a 16-segment wall motion index model for estimation of the LVEF. TIMI flow before and after pPCI was assessed by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands).
For comparison of the categorical variables, Pearson’s chi-square test was used. For continuous variables, the median and interquartile range were computed, and the groups were compared using the Mann-Whitney U test or the Kruskal-Wallis rank sum test. Kaplan-Meier estimates and curves were used to describe the cumulative survival, and the curves were compared using the log-rank test. Uni- and multivariate Cox regression analyses were used to model survival.
Results
The median symptom-to-door time was 95 minutes (interquartile range 55 to 180), the median door-to-balloon time was 111 minutes (interquartile range 91 to 135), and the median symptom-to-balloon time was 214 minutes (interquartile range 165 to 303). Most patients (n = 487, 71%) were transferred from a referral hospital.
The baseline characteristics stratified by the symptom-to-balloon time are listed in Table 1 . Patients with a longer symptom-to-balloon time were more likely to be older, to have hypertension, to have a higher heart rate and systolic blood pressure at admission, and to be treated with calcium antagonists and nitrates. Patients with a shorter symptom-to-balloon time were more likely to be current smokers.
| Variable | Symptom-to-Balloon Time (hrs) | p Value | ||
|---|---|---|---|---|
| <3 (n = 223) | 3–5 (n = 282) | ≥5 (n = 181) | ||
| Age (years) | 61 (52–68) | 62 (55–71) | 66 (54–75) | 0.002 |
| Men | 74% | 73% | 75% | 0.95 |
| Hypertension | 16% | 20% | 25% | 0.06 |
| Diabetes | 5% | 9% | 7% | 0.17 |
| Current smoking | 65% | 59% | 52% | 0.03 |
| Previous myocardial infarction | 11% | 10% | 11% | 0.89 |
| Previous angioplasty | 5% | 5% | 3% | 0.60 |
| Previous stroke | 2% | 4% | 3% | 0.55 |
| Heart rate (beats/min) | 70 (60–80) | 70 (60–84) | 80 (66–90) | <0.001 |
| Systolic blood pressure (mm Hg) | 130 (110–150) | 134 (120–150) | 140 (125–160) | <0.001 |
| Anterior myocardial infarction | 51% | 49% | 55% | 0.38 |
| Medical treatment | ||||
| Aspirin | 20% | 21% | 19% | 0.93 |
| β Blockers | 13% | 13% | 12% | 0.94 |
| Angiotensin-converting enzyme inhibitors | 7% | 7% | 12% | 0.10 |
| Calcium antagonists | 7% | 9% | 14% | 0.04 |
| Nitrate | 4% | 4% | 8% | 0.06 |
| Diuretics | 11% | 14% | 13% | 0.58 |
| Lipid-lowering drugs | 8% | 6% | 5% | 0.49 |
| Warfarin | 2% | 1% | 2% | 0.94 |
| No. of narrowed coronary arteries | 0.75 | |||
| 1 | 62% | 58% | 54% | |
| 2 | 23% | 28% | 28% | |
| 3 | 12% | 12% | 15% | |
| Left main | 3% | 2% | 3% | |
| Preangioplasty Thrombolysis In Myocardial Infarction flow | 0.13 | |||
| 0 | 60% | 66% | 70% | |
| 1 | 9% | 5% | 5% | |
| 2 | 13% | 16% | 14% | |
| 3 | 18% | 14% | 12% | |
A shorter symptom-to-balloon time was associated with greater rates of TIMI 3 flow after pPCI (early 86.5%, intermediate 80.9%, late 75.7%, p = 0.02). As shown in Figure 2 , a LVEF of ≤40% was an important predictor of 3-year mortality (hazard ratio [HR] 6.02, 95% confidence interval [CI] 3.68 to 9.85, p <0.001). A short symptom-to-balloon time was associated with a better predischarge LVEF (early 54%, range 45% to 60%; intermediate 50%, range 44% to 58%; late 50%, range 40% to 55%; p <0.001) and increased the likelihood of a subsequent LVEF >40% at hospital discharge (early 80.3%, intermediate 77.2%, late 66.9%, p = 0.006).
Figure 3 illustrates the association between the symptom-to-balloon time and 3-year survival. No long-term mortality difference was found between the early and intermediate groups (early vs intermediate, HR 1.05, 95% CI 0.58 to 1.90; early vs late, HR 2.42, 95% CI 1.38 to 4.23). We subsequently decided to analyze the early and intermediate groups as one group with a symptom-to-balloon time of <5 hours. A symptom-to-balloon time of ≥5 hours was associated with a significant mortality increase (HR 2.36, 95% CI 1.51 to 3.68, p <0.001). After controlling for baseline differences, only a trend toward a mortality difference remained (adjusted HR 1.55, 95% CI 0.98 to 2.44, p = 0.06). The independent baseline predictors of mortality were age (HR 1.08, per 1-year increase; 95% CI 1.06 to 1.11, p <0.001) and heart rate at admission (HR 1.19, per 10-beats/min increase; 95% CI 1.09 to 1.31, p <0.001). An analysis of a possible interaction among these 3 variables revealed an interaction between age and the symptom-to-balloon time (p = 0.047). In this model, allowing for the interaction, the symptom-to-balloon time was again significant (adjusted HR 2.44, 95% CI 1.31 to 4.54, p = 0.007). In patients aged ≤63 years (median age in entire DANAMI-2 cohort ), a symptom-to-balloon time of <5 hours was associated with greatly reduced mortality compared to a symptom-to-balloon time of ≥5 hours (3.2% vs 11.0% after 3 years, HR 3.60, 95% CI 1.39 to 9.34, p = 0.005). In contrast, in patients aged >63 years, a symptom-to-balloon time of <5 hours was associated with a smaller relative (but similar absolute) mortality reduction compared to a symptom-to-balloon time of ≥5 hours (15.7% vs 25.0% after 3 years; HR 1.67, 95% CI 1.01 to 2.76, p = 0.04).
The baseline characteristics stratified by the door-to-balloon time are listed in Table 2 . The patients with a longer door-to-balloon time were more likely to have hypertension and to be treated with β blockers, calcium antagonists, and nitrates, were less likely to be current smokers, and were less likely to have TIMI 0 flow before pPCI.
| Variable | Door-to-Balloon Time (min) | p Value | ||
|---|---|---|---|---|
| <90 (n = 160) | 90–105 (n = 133) | ≥105 (n = 393) | ||
| Age (years) | 62 (53–68) | 64 (55–73) | 63 (54–72) | 0.20 |
| Men | 72% | 71% | 76% | 0.36 |
| Hypertension | 14% | 15% | 24% | 0.01 |
| Diabetes | 4% | 6% | 9% | 0.11 |
| Current smoking | 65% | 57% | 57% | 0.21 |
| Previous myocardial infarction | 9% | 11% | 11% | 0.74 |
| Previous angioplasty | 4% | 4% | 5% | 0.87 |
| Previous stroke | 3% | 2% | 4% | 0.67 |
| Heart rate (beats/min) | 70 (60–80) | 74 (60–86) | 73 (61–86) | 0.26 |
| Systolic blood pressure (mm Hg) | 138 (116–150) | 131 (120–150) | 138 (120–155) | 0.86 |
| Anterior myocardial infarction | 51% | 46% | 53% | 0.37 |
| Medical treatment | ||||
| Aspirin | 15% | 21% | 22% | 0.17 |
| β Blockers | 8% | 13% | 15% | 0.06 |
| Angiotensin-converting enzyme inhibitors | 8% | 6% | 9% | 0.59 |
| Calcium antagonists | 5% | 9% | 12% | 0.05 |
| Nitrate | 1% | 5% | 6% | 0.04 |
| Diuretics | 9% | 11% | 15% | 0.09 |
| Lipid-lowering drugs | 8% | 5% | 6% | 0.49 |
| Warfarin | 1% | 1% | 2% | 0.25 |
| No. of narrowed coronary arteries | 0.53 | |||
| 1 | 63% | 62% | 55% | |
| 2 | 23% | 25% | 28% | |
| 3 | 13% | 12% | 14% | |
| Left main | 2% | 2% | 3% | |
| Preangioplasty Thrombolysis In Myocardial Infarction flow | 0.01 | |||
| 0 | 76% | 66% | 60% | |
| 1 | 5% | 8% | 7% | |
| 2 | 8% | 16% | 16% | |
| 3 | 11% | 11% | 18% | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


