Thymectomy
Jason Leonard Muesse
Shanda Haley Blackmon
DEFINITION
Thymectomy is defined as resection of the thymus gland.
The most common indications for thymectomy are myasthenia gravis and thymoma. Less common indications for thymectomy include thymic carcinoma, neuroendocrine tumors of the thymus, ectopic parathyroid glands, and thymic cysts.
Thymectomy has been most commonly performed through a median sternotomy, as described by Alfred Blalock. Alternate approaches include video-assisted thoracoscopic surgery (VATS), robotic-assisted thoracoscopic surgery (RATS), and transcervical approach.1
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of anterior mediastinal mass includes thymoma, thymic carcinoma, lymphoma, and germ cell tumor.
Thymomas have cytologic features of malignancy in less than 10% of cases. However, thymomas lacking malignant features cytologically can express malignant behavior by recurring locally and metastasizing even after resection; thus, the term “benign” thymoma should not be used.2
The most widely accepted and clinically applicable staging system for thymoma is the Masaoka staging system, which is based on microscopic or macroscopic invasion into mediastinal structures.3
PATIENT HISTORY AND PHYSICAL FINDINGS
One-third of patients with thymoma are asymptomatic.
Among patients symptomatic from thymoma, about 40% present with local symptoms directly related to the mass itself, including chest pain, cough, and dyspnea, but can include superior vena cava syndrome.2
The parathymic syndrome most commonly associated with thymoma is myasthenia gravis, which occurs in approximately 45% of patients with thymoma (reported ranges from 10% to 67%). Other associated autoimmune conditions include pure red cell aplasia and hypogammaglobulinemia, which can occur in up to 5% of people with thymoma.2
Approximately 10% to 15% of patients with myasthenia gravis have a thymoma.
Fevers, night sweats, and other constitutional symptoms may indicate lymphoma rather than thymoma.
IMAGING AND OTHER DIAGNOSTIC STUDIES
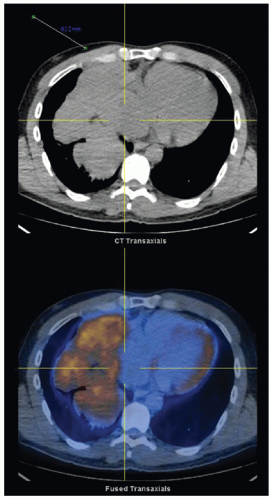
Computerized tomography is the most useful imaging modality for thymoma. Thin-slice cuts will improve preoperative planning.
Thymomas can have variable uptake on positron emission tomography, so it is not routinely recommended. Magnetic resonance imaging can also provide helpful information about invasion into vessels or adjacent structures (FIG 1).4
Masses larger than 5 cm (not including thymus gland itself) in maximal diameter or those invading mediastinal structures either warrant specialized surgical planning or possibly enrollment into a neoadjuvant treatment protocol.5
Fine needle aspiration or core biopsy of the anterior mediastinal mass is recommended preoperatively in most cases to rule out lymphoma or germ cell tumor. If the biopsy is constituent with thymoma, resection of the entire thymic gland is indicated.4
Myasthenia gravis can be diagnosed with serum testing for the acetylcholine receptor antibody or through clinical improvement in symptoms with administration of edrophonium chloride.
SURGICAL MANAGEMENT
Preoperative Planning
The decision to perform thymectomy for treatment of myasthenia gravis must involve the patient, the treating neurologist, the anesthesiologist, and the thoracic surgeon. Preoperative optimization of the patient’s myasthenia gravis symptoms with pyridostigmine, steroids, and in some cases, plasmapheresis is critical.
Special anesthetic requirements may be necessary for patients with myasthenia gravis, taking care to avoid anticholinergics. The anesthetic team should be notified preoperatively of the patient’s medical condition and the severity of their symptoms in appropriate time to make accommodations.6
All patients who will undergo single-lung ventilation (VATS or RATS) should have preoperative pulmonary function testing to ensure they will be able to tolerate single-lung ventilation during resection. If the patient is deemed unable to tolerate single-lung ventilation, resection via sternotomy or transcervical approach is indicated. All patients with myasthenia gravis need pulmonary function testing preoperatively.
Central venous access should be obtained prior to beginning procedure.
A sternal saw should always be present in the operating suite and readily available in the event emergent conversion to sternotomy for bleeding is necessary.
Positioning
For median sternotomy or transcervical approach, a single lumen endotracheal tube is adequate.
For VATS or robotic-assisted thoracoscopic approach, we strongly prefer the use of double lumen endotracheal tube placement to single lumen endotracheal tube with bronchial blockers. If the patient needs to be repositioned intraoperatively, we feel that the double lumen endotracheal tube is much less likely to be dislodged than a bronchial blocker and provides more reliable lung isolation.
We confirm the placement of the double lumen endotracheal tube with bronchoscopy before positioning the patient.
Each time the patient is repositioned, proper placement of the double lumen endotracheal tube is confirmed with bronchoscopy.
THYMECTOMY BY STERNOTOMY
Positioning
Place patient in supine position.
We prefer arms tucked at the patient’s side and use an axillary roll placed laterally under the scapulae.
Use clippers to remove hair from sternal notch to below xiphoid process. Prep and drape skin in sterile fashion from the sternal notch to below xiphoid process.
Boundaries of Dissection
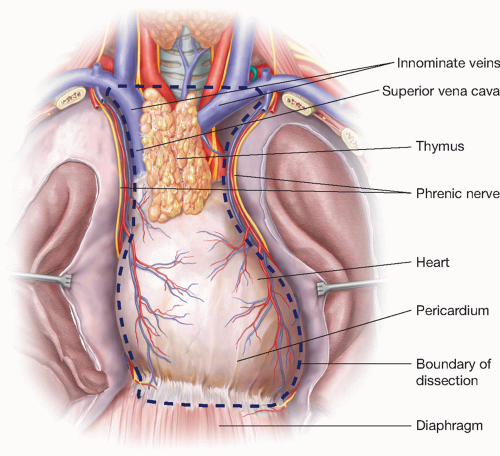
The boundaries of dissection for radical thymectomy are the innominate veins superiorly, the phrenic nerves bilaterally, and the diaphragm inferiorly (FIG 2).
Preservation of the phrenic nerve is of the upmost importance and it should never be sacrificed unless the diagnosis of thymic carcinoma is confirmed and tumor grossly invades the nerve. Even in these cases, the surgeon must consider the implications of diaphragmatic paralysis on a patient who may need adjuvant chemotherapy and radiation. Both phrenic nerves should never be divided.1
With invasive thymoma, involved structures should be resected en bloc with the thymic mass at the time of initial operation, always sparing at least one phrenic nerve.7
Mark areas of dissection for invasive thymoma with surgical clips to help direct future radiation therapy.7
Median Sternotomy Incision
Partial or “mini-sternotomy” can be used as long as entirety of thymus tissue can accessed and phrenic nerves visualized bilaterally. Visualization must not be sacrificed for cosmesis (FIG 3).7
Incise skin from 1.5 cm below the sternal notch to level of 4th or 5th intercostal space for partial sternotomy or to xiphoid process for full sternotomy.
Use a sternal saw to separate the sternum down the midline.
Mind adhesions that could be present from the underlying thymoma itself or from previous sternotomy.
Right Inferior Pole Dissection
The order of dissection with regard to thymectomy by sternotomy is surgeon preference, with regard to starting on the right or left or from the superior or inferior. We choose to start at the right inferior pole for both open and minimally invasive approaches.
The right phrenic nerve is identified.
The right inferior pole is identified and dissected from the pericardial fat pad, dividing attachments to nonthymic tissue.
Arterial blood supply to the inferior pole is from the pericardiophrenic branches. These small vessels are well suited for ligation with a bipolar energy source such as LigaSure (Covidien, Mansfield, Massachusets) or an ultrasonic vesselsealing device such as the Harmonic Ace (Ethicon Endo-Surgery, Blue Ash, Ohio). These devices are also useful for dividing the mediastinal pleura and connecting tissue.
The mediastinal pleura is incised 1 cm medial to the right phrenic nerve and division is carried cranially along the lateral aspect of the thymus toward the superior pole. Be mindful of lateral energy transfer from electrocautery or bipolar energy source that could damage the phrenic nerve.
The major arterial blood supply to the thymus is lateral, from branches of the internal mammary artery. These vessels can be tied, clipped, or divided with advanced vessel-sealing devices as mentioned earlier.
Right Superior Pole Dissection
Continue dissection of the mediastinal pleura cranially toward the right superior pole.
Gently use the divided thymic tissue as a handle for downward retraction to expose the right superior pole.
The arterial blood supply to the superior pole branches off the inferior thyroid artery. These vessels can be divided between ties or clips or using advanced vessel-sealing devices.
Divide the right thyrothymic ligament between clamps or using an advanced vessel-sealing device. Once vessels or tissue attached to the superior pole are divided, they can retract cranially, making gaining control of bleeding difficult.
Occasionally, a vein draining into the right innominate vein may be encountered. Divide it if present.
Left Inferior Pole Dissection
Redirect attention to the right inferior pole. Divide the mediastinal pleura from the right lateral pole to the left lateral pole just above the level of the diaphragm, taking care to include all inferior thymic tissue with the thymus.
Identify the left phrenic nerve and avoid damage to it when dividing laterally across the inferior aspect of the thymic tissue.
Dissect the left inferior pole away from the pericardial fat pad and divide the left inferior pole from its attachments.
Incise the mediastinal pleura 1 cm medially from the left phrenic nerve and continue division cranially.
Divide the left lateral branches of the internal mammary artery.
Left Superior Pole Dissection
Gently use divided thymic tissue for downward retraction.
Proceed with division of lateral attachments of thymic tissue toward the left superior pole. Divide remaining attachments of the left superior pole, including branches of the left inferior thyroid artery supplying it.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree