To compare the long-term efficacy and safety of sirolimus-eluting stents (SES) to those of bare-metal stents (BMS) for ST-segment elevation myocardial infarction, outcomes were assessed in 310 patients (mean age age 59 ± 11 years, 78% men) included in the randomized MISSION! Intervention Study: A Prospective Randomised Controlled Trial to Evaluate the Efficacy of Drug-Eluting Stents Versus Bare-Metal Stents for the Treatment of Acute Myocardial Infarction after a median follow-up period of 38 months. All patients were treated with aspirin (lifelong) and clopidogrel for 1 year after stent implantation. Except for a significant difference between reference vessel diameters (SES 2.76 mm vs BMS 2.92 mm, p = 0.02), there were no significant differences in baseline and angiographic characteristics between the treatment groups (158 SES, 152 BMS). A significant difference between SES and BMS patients for all revascularization end points was found after the first year of follow-up. However, at 3 years of follow-up, although there was still a trend toward better clinical outcomes in SES-treated patients, differences were no longer significant (death 4.4% vs 6.6%, p = 0.41; target vessel–related myocardial infarction 2.5% vs 4.6%, p = 0.32; target vessel revascularization 8.9% vs 15.8%, p = 0.06), target lesion revascularization 6.3% vs 12.5%, p = 0.06; and target vessel failure 12.0% vs 19.7%, p = 0.06). Three cases of very late (definite) stent thrombosis were observed in the SES group (1.9%) versus none in the BMS group (p = 0.14). In conclusion, the significant SES benefit (compared to BMS) in patients with ST-segment elevation myocardial infarctions at 1-year follow-up in terms of target vessel revascularizations decreased to some extent because of more similar target vessel revascularization rates during the 2 subsequent years. Rates of death and nonfatal recurrent MI remained comparable.
This randomized prospective study was designed to evaluate angiographic outcomes and clinical efficacy of third-generation bare-metal stents (BMS) compared to those seen with sirolimus-eluting stents (SES) in patients with ST-segment elevation myocardial infarction (STEMI). After the midterm (12-month) angiographic and clinical results, the present study evaluated clinical outcomes after 3 years of follow-up from the index event.
Methods
The MISSION! Intervention Study: A Prospective Randomised Controlled Trial to Evaluate the Efficacy of Drug-Eluting Stents Versus Bare-Metal Stents for the Treatment of Acute Myocardial Infarction (Current Controlled Trials number ISRCTN62825862) was a single-center, single-blind, randomized prospective study to evaluate clinical and 9-month angiographic results in patients with STEMI treated with either BMS or SES. The study protocol was approved by the institutional ethics committee. Written informed consent was obtained from all patients before enrollment and before follow-up catheterization. Patients and operators performing follow-up were blinded to treatment assignment. During the study period, all patients were treated according to the institutional STEMI protocol, which included standardized outpatient follow-up.
The study design, and methods have been described in detail previously. In brief, consecutive patients with de novo coronary lesions were eligible for participation if symptoms of STEMI started <9 hours before arrival at the catheterization laboratory and electrocardiography demonstrated a STEMI. Exclusion criteria have been detailed previously but in summary consisted of any “off-label” indication other than STEMI. Randomization to treatment with a BMS (Vision; Guidant Corporation, Indianapolis, Indiana) or SES (Cypher; Cordis Corporation, Miami Lakes, Florida) was performed in a 1:1 ratio.
Before the procedure, all patients received aspirin 300 mg, clopidogrel 300 to 600 mg, and an intravenous bolus of abciximab 25 μg/kg, followed by a continuous infusion of 10 μg/kg/min for 12 hours. At start of the procedure, heparin 5,000 IU was given. Lesions were treated according to current interventional practice.
The 2 treatment groups received dual-antiplatelet therapy for an equal treatment duration. Aspirin 100 mg/day was prescribed indefinitely and clopidogrel 75 mg/day for 12 months. Patients were seen at the outpatient clinic at 30 days and 3, 6, and 12 months according to the MISSION! care program. During follow-up, patients were treated with β-blocking agents, statins, and angiotensin-converting enzyme inhibitors or angiotensin II blockers. Follow-up angiography was performed at 9 months.
Long-term follow-up data from each patient were documented prospectively in the electronic patient file and data management system of Leiden University Medical Center (EPD-Vision 6.01). Data were recorded after 3 years by patient visits at the outpatient clinic or, if not possible, by telephone inquiry. When a patient visit took place at another hospital, specific data inquiry was performed after obtaining written consent of the patient.
The end points of the present study were death, myocardial infarction, target vessel revascularization, target lesion revascularization, target vessel failure, and stent thrombosis. All deaths were defined as cardiac unless unequivocally proved noncardiac. Myocardial infarction during follow-up was defined as a troponin T increase >0.03 μg/L with symptoms or percutaneous coronary intervention (PCI), an increase in troponin T >0.15 μg/L after coronary artery bypass grafting, or a subsequent increase in troponin T >25% after recent myocardial infarction in the presence of symptoms or repeat PCI or the development of new Q waves on electrocardiography. Infarctions were categorized as spontaneous or procedure related (nonindex procedure).
Target vessel and target lesion revascularization were defined as any revascularization procedure of the target vessel or target lesion, respectively. Target vessel failure was defined as the composite of cardiac death or recurrent nonfatal myocardial infarction attributable to the target vessel or any revascularization procedure of the target vessel. If events could not unequivocally be attributed to a nonculprit vessel, they were considered culprit vessel related.
Stent thrombosis was defined as definite, probable, or possible (the composite of these being total stent thrombosis), further subdivided into acute (≤1 day), subacute (>1 day to ≤1 month), late (>1 month to ≤1 year), and very late (>1 year) stent thrombosis, according to the Academic Research Consortium definition. All clinical events were adjudicated by a clinical events committee whose members were blinded to the assigned stent type.
Because this study was planned as a follow-up investigation of the MISSION! Intervention Study, sample-size calculations were done for the original purpose only. Analyses were conducted according to the intention-to-treat principle. Continuous data are expressed as mean ± SD or as median (interquartile range); dichotomous data are presented as numbers and percentages. All continuous variables were compared between the treatment groups with Student’s t test or, in the case of a non-Gaussian distribution, with a nonparametric test. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test as appropriate. Event rates over time were analyzed using the Kaplan-Meier method with corresponding log-rank tests for differences in distribution between the curves.
The effect of a reference diameter ≥3 mm on the risk for stent thrombosis was estimated by multivariate Cox regression analysis with treatment group as sole covariate. The rationale to conduct analysis this way was as follows: other potential (known and unknown) confounders have already been accounted for because of the randomized design of this study. Adding variables to the multivariate analysis after randomization may reduce comparability between the treatment groups. Therefore, only variables that were known to be different from baseline, such as stent type and reference vessel diameter, were entered into the multivariate model. All p values were 2 sided, and a p value <0.05 was considered statistically significant. All analyses were conducted using SPSS version 16.0 (SPSS, Inc., Chicago, Illinois).
Results
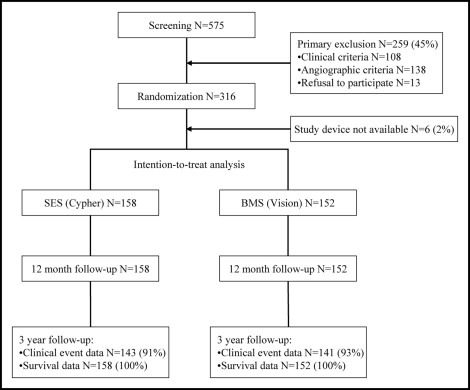
A total of 316 patients with STEMI were enrolled in the study ( Figure 1 ). Six patients were subsequently excluded because the assigned study stent was not available, and 310 patients (152 assigned to BMS and 158 assigned to SES) were included in the analysis. Baseline characteristics of the study population are listed in Table 1 . With exception of a slightly larger reference diameter in the BMS group, the groups were comparable. One patient crossed over from SES to BMS because of the inability to cross the lesion with the SES. No patients were lost to follow-up, and all patients were contacted ( Figure 1 ). Complete clinical data were available for 91% of the patients assigned to the SES group and for 93% of the patients assigned to the BMS group.
| Characteristic | SES | BMS | p Value |
|---|---|---|---|
| (n = 158) | (n = 152) | ||
| Age (years) | 59.2 ± 11.2 | 59.1 ± 11.6 | 0.99 |
| Men | 118 (74.7%) | 123 (80.9%) | 0.19 |
| Diabetes mellitus | 20 (12.7%) | 10 (6.6%) | 0.07 |
| Current smoker | 84 (53.2%) | 85 (55.9%) | 0.63 |
| Hypercholesterolemia † | 37 (23.4%) | 25 (16.4%) | 0.13 |
| Hypertension ‡ | 48 (30.4%) | 39 (25.7%) | 0.36 |
| Family history of coronary artery disease | 73 (46.2%) | 60 (39.5%) | 0.23 |
| Previous myocardial infarction | 7 (4.4%) | 5 (3.3%) | 0.60 |
| Previous percutaneous coronary intervention | 4 (2.5%) | 1 (0.7%) | 0.37 |
| Previous coronary artery bypass grafting | 1 (0.6%) | 1 (0.7%) | 1.00 |
| Symptom onset to first electrocardiogram (minutes) | 88 (47–153) | 106 (71–151) | 0.11 |
| Symptom onset to balloon inflation (minutes) | 183 (133–258) | 195 (153–257) | 0.19 |
| Target coronary artery | |||
| Left | 87 (55.1%) | 83 (54.6%) | |
| Right | 40 (25.3%) | 51 (33.6%) | 0.09 |
| Left circumflex | 31 (19.6%) | 18 (11.8%) | |
| Multivessel disease | 56 (35.4%) | 50 (32.9%) | 0.64 |
| Thrombolysis In Myocardial Infarction flow grade before | |||
| 0 | 96 (60.8%) | 90 (59.2%) | |
| 1 | 18 (11.4%) | 15 (9.9%) | 0.87 |
| 2 | 20 (12.6%) | 24 (15.8%) | |
| 3 | 24 (15.2%) | 23 (15.1%) | |
| Maximal creatinine phosphokinase (U/L) | 1,844 (863–3,413) | 2,079 (1,012–3,792) | 0.25 |
| Quantitative coronary angiography preprocedure | |||
| Lesion length (mm) | 13.9 ± 5.6 | 15.0 ± 8.6 | 0.47 |
| Reference diameter (mm) | 2.76 ± 0.54 | 2.92 ± 0.56 | 0.02 ⁎ |
| Minimal luminal diameter (mm) | 0.21 ± 0.35 | 0.27 ± 0.41 | 0.19 |
| Stenosis (% luminal diameter) | 91.0 ± 13.6 | 92.5 ± 12.4 | 0.35 |
† Total cholesterol ≥190 mg/dl or previous pharmacologic treatment.
‡ Blood pressure ≥140/90 mm Hg or previous pharmacologic treatment.
Clopidogrel was used for up to 12 months by 93% of patients (147 of 158, with 156 patients alive at follow-up) in the SES group and by 96% of patients (146 of 152, with 148 patients alive at follow-up) in the BMS group (p = 0.24). Aspirin treatment was continued by all patients during the entire follow-up of 3 years except when oral anticoagulation was indicated (n = 39). Twenty-one patients (11 BMS, 10 SES) used clopidogrel for >1 year. Reasons for prolongation of clopidogrel treatment were in most cases (n = 17) the occurrence of an in-stent restenosis or stent thrombosis, and in 4 cases because of patient-doctor miscommunication. All patients who experienced target lesion revascularization and/or stent thrombosis <1 year after myocardial infarction used clopidogrel at the time the first event took place. This was true for patients in the 2 treatment groups.
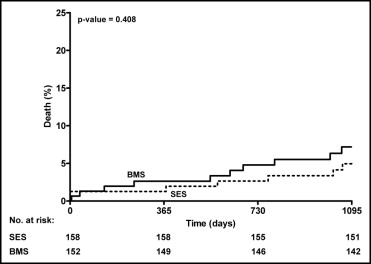
Clinical outcomes data at long-term follow-up are listed in Table 2 . Compared to the previously reported midterm results, 11 additional deaths occurred, 5 in the SES group and 6 in the BMS group (p = NS). About half of these additional deaths were noncardiac (6 of 11 [55%], all related to malignancies). Kaplan-Meier estimates of the cumulative incidence of all-cause death for the SES and BMS groups are shown in Figure 2 . The 2 treatment groups demonstrated a similar probability of all-cause death over the years (log-rank test p = 0.41).
| Event | 12-Month Outcomes | 3-Year Outcomes | ||||
|---|---|---|---|---|---|---|
| SES | BMS | p Value | SES | BMS | p Value | |
| (n = 158) | (n = 152) | (n = 158) | (n = 152) | |||
| Death | 2 (1.3%) | 4 (2.6%) | 0.44 | 7 (4.4%) | 10 (6.6%) | 0.41 |
| Noncardiac | — | 2 (1.3%) | 0.24 | 3 (1.9%) | 5 (3.3%) | 0.68 |
| Cardiac | 2 (1.3%) | 2 (1.3%) | 1.00 | 4 (2.5%) | 5 (3.3%) | 0.95 |
| Recurrent myocardial infarction † | 9 (5.7%) | 14 (9.2%) | 0.24 | 12 (7.6%) | 17 (11.2%) | 0.28 |
| Spontaneous | 2 (1.3%) | 3 (2.0%) | 0.68 | 5 (3.2%) | 7 (4.6%) | 0.51 |
| Target vessel related | 2 (1.3%) | 3 (2.0%) | 0.68 | 4 (2.5%) | 7 (4.6%) | 0.32 |
| Procedure related | 7 (4.4%) | 11 (7.2%) | 0.29 | 7 (4.4%) | 11 (7.2%) | 0.29 |
| Target vessel related | 2 (1.3%) | 6 (3.9%) | 0.17 | 2 (1.3%) | 6 (3.9%) | 0.17 |
| Revascularization procedure † | 19 (12.0%) | 35 (23.0%) | 0.01 ⁎ | 28 (17.7%) | 39 (25.7%) | 0.09 |
| Percutaneous coronary intervention | 17 (10.8%) | 30 (19.7%) | 0.03 ⁎ | 26 (16.5%) | 33 (21.7%) | 0.24 |
| Coronary artery bypass grafting | 2 (1.3%) | 5 (3.3%) | 0.28 | 3 (1.9%) | 8 (5.3%) | 0.11 |
| Target vessel revascularization † | 8 (5.1%) | 20 (13.2%) | 0.01 ⁎ | 14 (8.9%) | 24 (15.8%) | 0.06 |
| Percutaneous coronary intervention | 6 (3.8%) | 17 (11.2%) | 0.01 ⁎ | 11 (7.0%) | 20 (13.2%) | 0.07 |
| Coronary artery bypass grafting | 2 (1.3%) | 3 (2.0%) | 0.68 | 3 (1.9%) | 6 (3.9%) | 0.46 |
| Target lesion revascularization † | 5 (3.2%) | 17 (11.2%) | 0.006 ⁎ | 10 (6.3%) | 19 (12.5%) | 0.06 |
| Percutaneous coronary intervention | 3 (1.9%) | 14 (9.2%) | 0.005 ⁎ | 7 (4.4%) | 15 (9.9%) | 0.06 |
| Coronary artery bypass grafting | 2 (1.3%) | 3 (2.0%) | 0.68 | 3 (1.9%) | 6 (3.9%) | 0.46 |
| Clinically driven | 4 (2.5%) | 12 (7.9%) | 0.03 ⁎ | 9 (5.7%) | 14 (9.2%) | 0.28 |
| Target vessel failure | 11 (7.0%) | 23 (15.1%) | 0.02 ⁎ | 19 (12.0%) | 30 (19.7%) | 0.06 |
| Stent thrombosis | ||||||
| Definite | 1 (0.6%) | 1 (0.7%) | 1.00 | 4 (2.5%) | 1 (0.7%) | 0.39 |
| Probable | 1 (0.6%) | 2 (1.3%) | 0.97 | 1 (0.6%) | 2 (1.3%) | 0.97 |
| Possible | — | — | — | 1 (0.6%) | 1 (0.7%) | 1.00 |