Three-Dimensional Transesophageal Echocardiography
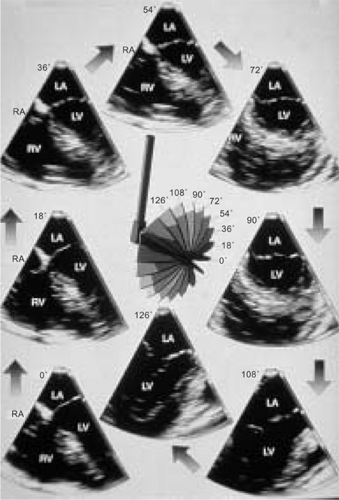
Sequential multiplane two-dimensional transesophageal echocardiographic (2D TEE) images provide a detailed assessment of cardiac lesions and are ideal for three-dimensional transesophageal echocardiographic (3D TEE) reconstruction. The first study using a multiplane 2D TEE probe to perform 3D reconstruction was published in 1992, from our laboratory. Basically, a selected area of interest was placed in the center of the imaging sector and images acquired by rotating the transducer from 0° to 180° in small increments of 1° to 3°, taking care to keep the probe fixed in one position and the patient lying still. The acquired images were then transferred to an offline computer (TomTec Company, Munich, Germany) for subsequent 3D reconstruction. A more recent innovation enables 3D reconstruction on the same ultrasound system that is used to acquire sequential 2D images. Also, the time taken to reconstruct a 3D image has been reduced to about 1 minute. To minimize artifacts in the 3D TEE reconstructed images, it is important to acquire images only in a particular phase of respiration, and at comparable RR intervals. This is often done by setting up a gating window utilizing an online histogram based on the patient’s heart rate.
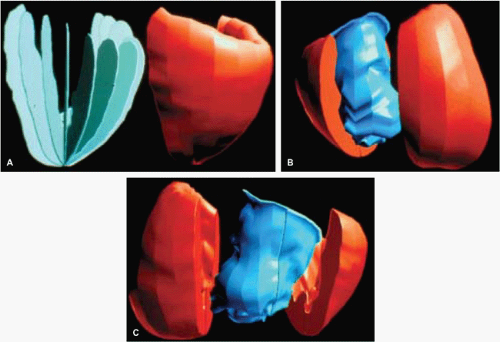
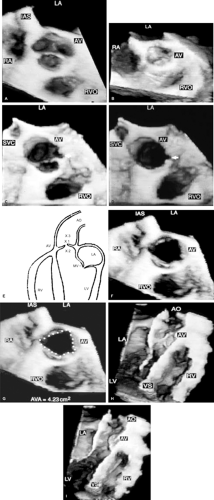
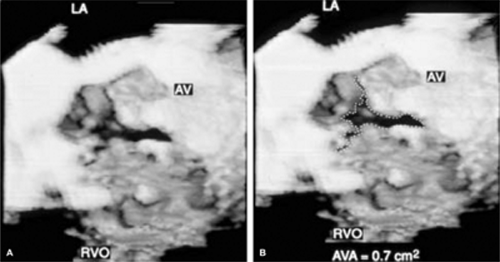
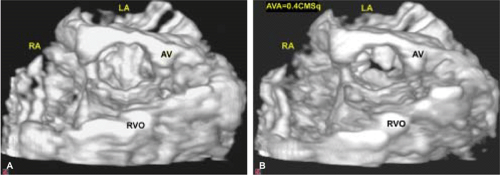
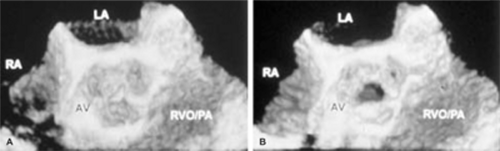
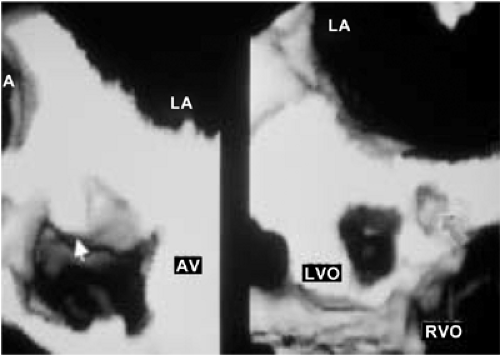
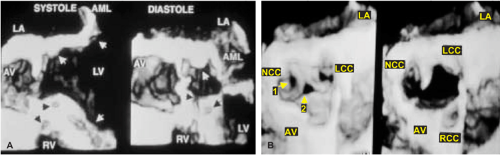
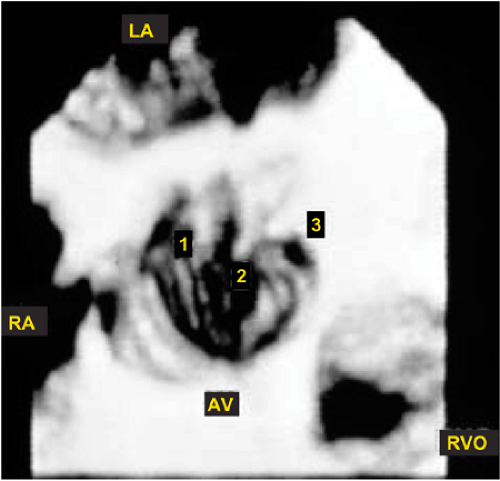
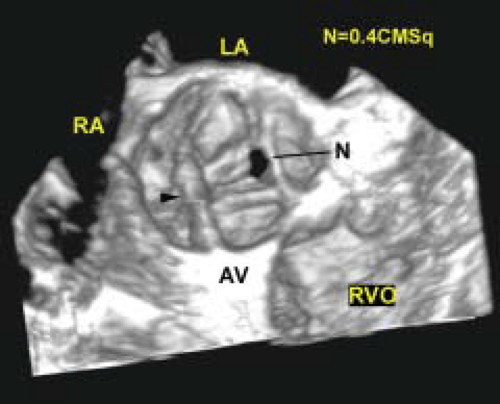
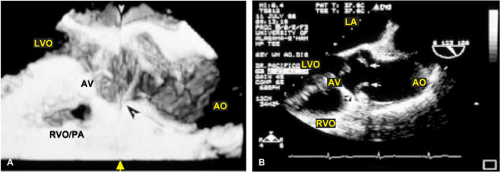
An important clinical application of 3D TEE reconstruction is the assessment of left ventricular volumes and ejection fraction without making an assumption regarding the geometric shape of the left ventricle (LV). This improves accuracy and reduces intra- and inter-observer variability, an important limitation of 2D echocardiography. An early application of 3D TEE was accurate assessment of aortic valve morphology and orifice area in patients with aortic stenosis undergoing 2D TEE. The 3D volumetric data set permits short axis sections across the aortic valve at any desired angle, resulting in the identification of the smallest aortic valve orifice area for planimetry, usually located at the tip of the aortic valve. With 2D TEE, the probe is moved up and down the esophagus to identify the smallest orifice. 3D TEE has the advantage of being able to examine any planes that would increase the likelihood that the imaging plane is exactly parallel to the aortic orifice, maximizing the accuracy of stenotic orifice area measurement.
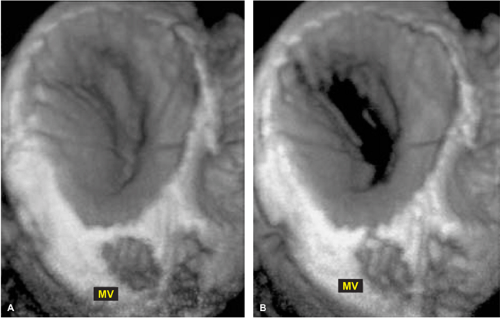
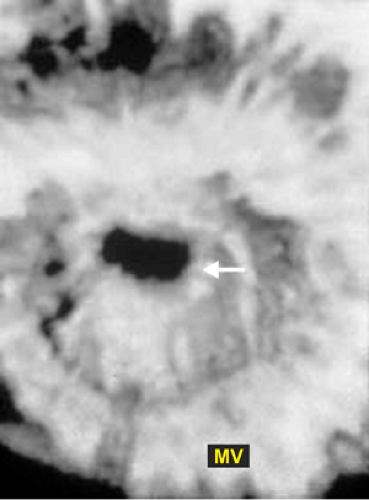
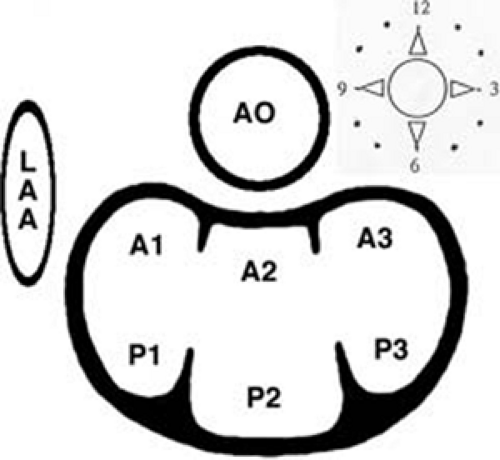
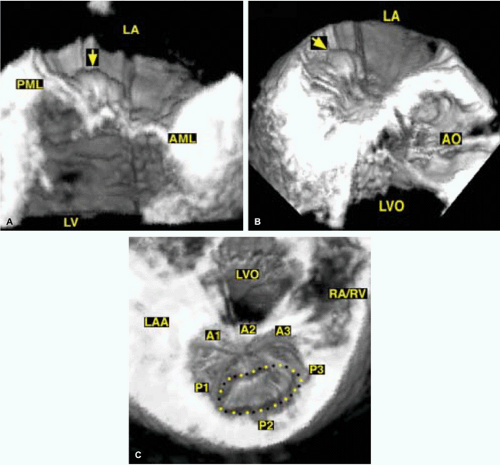
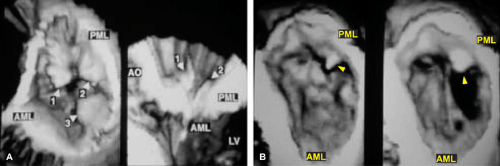
3D TEE is superior to 2D TEE in the detection of individual scallop/segment prolapse of the mitral valve. Accurate identification of scallop or segment prolapse and its extent is crucial when considering patients for mitral valve repair. With 3D TEE, short-axis cuts can be taken at any level and the atrial surfaces of both leaflets viewed en face. Therefore, individual scallop or segment prolapse and its extent can be easily assessed. This information is important for the surgeon who may find it difficult to estimate the true extent of any scallop or segment prolapse in a heart devoid of blood. As discussed in Chapter 2, multiplane 2D TEE has important limitations in the assessment of individual scallop or scallop prolapse.
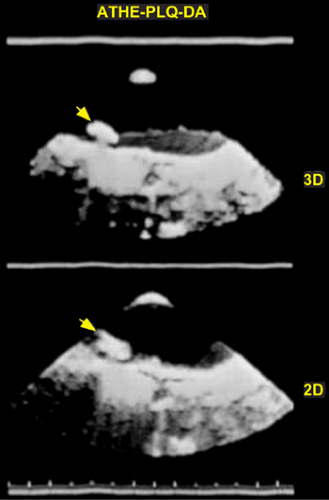
3D TEE is also superior to 2D TEE in assessing the dimensions of intracardiac masses such as infective vegetations, thrombi, atherosclerotic plaques, tumors, and intracardiac defects such as atrial septal defects. In several instances, the maximum size of these lesions has been underestimated by multiplane 2D TEE, as compared to 3D TEE and surgery or pathologic measurements. Using multiplane 2D TEE, the maximal dimension of a lesion can be obtained only if its long axis lies exactly parallel to the ultrasonic beam as it rotates from 0° to 180°; otherwise the maximum linear dimension will be underestimated. On the other hand, with 3D TEE, any imaging plane can be interrogated in the search for the maximum lesion dimension. 3D TEE also permits accurate assessment of volumes of intracardiac masses. Unlike multiplane 2D TEE, 3D TEE enables en face viewing of intracardiac defects and openings in congenital membranes such as cor triatriatum sinister, permitting accurate assessment of their shape and size. These aspects are of practical clinical importance. For instance, the size of infective vegetations correlates with embolic potential and patient prognosis and information on the exact size of an atrial septal defect is important during catheter closure of the defect.
Another example of the utility of 3D TEE in providing anatomic definition has been the assessment of Lambl’s excrescences on the aortic valve. In a few patients studied by us, more Lambl’s excrescences were found using 3D TEE than using 2D TEE. We have also found 3D TEE to be useful in providing a more secure diagnosis of an aortic valve papillary fibroelastoma. Multiple small projections from the tumor and bright echoes in the middle of the stalk, consistent with fronds and a collagenous central core, both pathologically characteristic of a fibroelastoma, were better seen using 3D TEE than using multiplane 2D TEE.
3D TEE has supplemented multiplane 2D TEE in the assessment of aortic dissection, specifically in patients in whom it is difficult to distinguish a dissection flap from an imaging artifact. In these instances, misdiagnosis has occurred. With 3D TEE, the dissection flap is seen in 3D as a sheet of tissue and not a linear structure, improving the specificity of diagnosis as compared to 2D TEE. Because 3D TEE provides en face views of prosthetic valves, abnormalities such as suture dehiscence and abscesses can be well visualized and their exact location in relation to the prosthesis can be accurately identified. Because the orientation of TEE images does not correspond to the surgeon’s view in the operating room, we have found it useful to describe images in relation to a surgical clock corresponding to the surgeon’s field of view as he stands on the right side of the patient. When the surgeon looks at the mitral prosthesis, the left atrial appendage is positioned at 9 o’clock or 10 o’clock, with the aorta at 12 o’clock. These landmarks can be used by the echocardiographer to describe the exact location of abnormalities such as suture dehiscence. For the aortic prosthesis, the clock is arranged with the atrial septum and left coronary artery at 7 o’clock and 11 o’clock positions, respectively. Important landmarks during the surgeon’s exposure of the tricuspid valve and right atrium are the inferior and superior vena cavae at the 4 o’clock and 8 o’clock to 9 o’clock, respectively.
3D TEE has also been useful in evaluating patients with left ventricular pseudoaneurysms. In one such patient with associated severe mitral regurgitation, 3D TEE clearly showed localized distortion of the mitral annulus produced by the pseudoaneurysm. Because there was no other obvious etiology to explain the presence of severe mitral regurgitation, it was decided not to repair or replace the mitral valve. Decompression of the pseudoaneurysm following repair normalized the mitral annulus, resulting in almost total abolition of mitral regurgitation. Therefore, in this patient, 3D TEE identified a localized distortion of the mitral annulus responsible for the presence of severe mitral regurgitation.
When examining proximal and/or mid coronary arteries by multiplane 2D TEE, only small segments are usually visualized in any given view as the ultrasonic 2D plane moves in and out of the plane of the coronary arteries. This problem is alleviated by 3D TEE since all visualized segments are contained within the 3D volumetric data set, which can be cropped suitably to bring longer segments of the coronary arteries into view. Also, sections can be taken exactly parallel to the short axis of a given coronary artery to ascertain the presence or absence of significant stenosis. With multiplane 2D TEE, an eccentrically located plaque or an oblique plane can create an impression of severe stenosis when none may be present. Therefore, 3D TEE is a useful adjunct to multiplane 2D TEE in the evaluation of coronary arteries for stenosis.
In conclusion, in many patients, 3D TEE provides important information beyond that provided by 2D TEE.
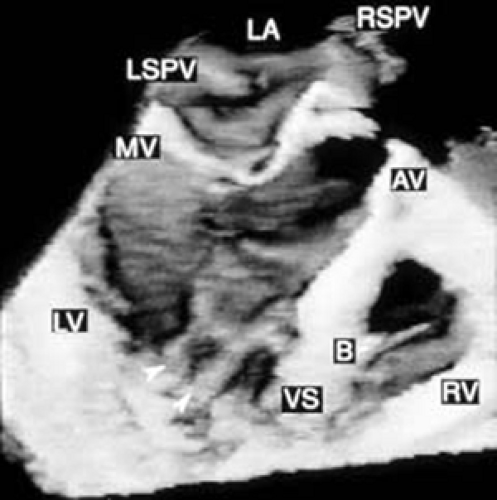 FIGURE 12.6. Three-dimensional reconstruction of the ventricle and right ventricle in the same patient shown in Figure 12.5. Arrowheads point to papillary muscles in the left ventricle. B, moderator band in the right ventricle; AV, aortic valve; LA, left atrium; LSPV, left superior pulmonary vein; LV, left ventricle; MV, mitral valve; RSPV, right superior pulmonary vein; RV, right ventricle; VS, ventricular septum. (Reproduced with permission from Nanda NC, Roychoudhury D, Chung SM, et al. Quantitative assessment of normal and stenotic aortic valve using transesophageal three-dimensional echocardiography. Echocardiography 1994;11:617–625. ) |
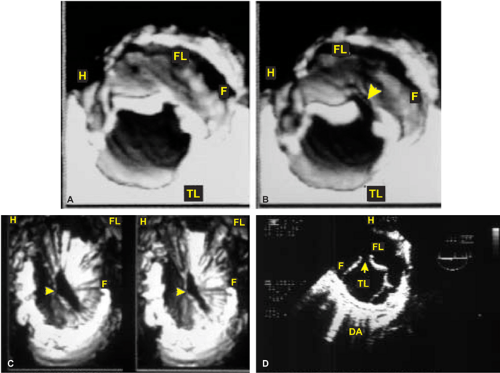
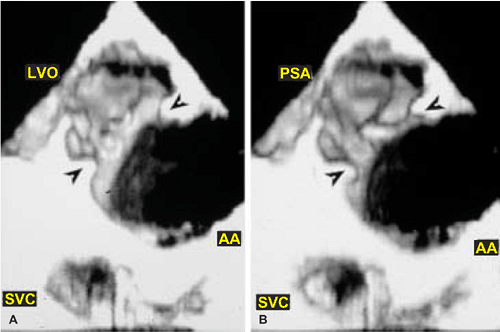 FIGURE 12.21. Transesophageal three-dimensional reconstruction in a patient with aortic arch (AA) injury from blunt trauma. A,B.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|