The present study aimed to investigate geometric remodeling of the mitral valve (MV) and to identify the geometric determinants of mitral regurgitation (MR) severity in patients with significant MR secondary to a rheumatic or prolapse etiology. We studied 90 consecutive patients in normal sinus rhythm, including 70 patients showing significant MR (52 with prolapsed/flail and 18 with rheumatic MV) and 20 controls with normal MV without MR. A full volume image was acquired using transesophageal echocardiography, and geometric analysis of the MV leaflet was performed with dedicated software. Areas of the MV annulus and the anterior and posterior leaflets were larger in the rheumatic and prolapsed MV than in the normal controls. No difference was found between the rheumatic and prolapsed MR in those parameters, except that the posterior leaflet area was smaller in rheumatic MR than in prolapsed MR. The leaflet to annulus area ratio was lower and the anterior to posterior leaflet area ratio was higher in the rheumatic MR group than in the prolapsed MR group. A large anteroposterior annulus diameter and small posterior leaflet tenting angle were independently associated with the effective regurgitant orifice area in rheumatic MV, although the leaflet to annulus area ratio was independently associated with the effective regurgitant orifice area in the prolapsed MV. In conclusion, similarities and differences in geometric MV remodeling exist between rheumatic and prolapsed MR. The knowledge of those quantitative differences could open the way to precise planning of surgery tailored to the underlying pathologic entity.
Mitral valve (MV) repair has been recommended over replacement for most patients with severe chronic mitral regurgitation (MR) who require surgery. Myxomatous MV degeneration causing a prolapsed or flailed MV is a typical candidate for MV repair. Various surgical techniques can be used, including annuloplasty, quadrangular or triangular resection, and artificial chordae formation. Rheumatic valvulitis, which leads to thickening, commissure fusion, and motion limitations of the MV, is another major cause of MR. Myxomatous and rheumatic MV differ in their morphologic characteristics. Previous studies have reported that similarities and differences exist in the activated gene expressed in the MV between the 2 pathologic entities, which can result in their unique morphologic features. However, geometric analyses of the morphologic remodeling occurring in these pathologic entities have not been performed. Both prolapsed and rheumatic MV can be successfully managed by MV repair ; however, the surgical methods used should reflect the underlying pathology. In certain clinical situations, MV repair of rheumatic MR is associated with a high incidence of MV reoperation or high long-term morbidity. Analysis of the structural differences between the 2 pathologic entities might be an essential first step toward identifying the optimal surgical methods. We aimed to investigate geometric remodeling of the MV secondary to a rheumatic and prolapse etiology in patients with significant MR, and to identify the geometric determinants of MR severity in these pathologic entities using 3-dimensional (3D) transesophageal echocardiography.
Methods
The present study prospectively enrolled 90 consecutive patients (mean age 55 ± 14 years; 43 women) in normal sinus rhythm, including 70 patients with significant MR (52 with prolapsed/flail and 18 with rheumatic MV) and 20 controls with a normal MV without MR. Of the 52 patients with a prolapsed/flail MV, the pathologic segments were only in the posterior leaflet, only in the anterior leaflet, and in both the anterior and the posterior leaflets in 34, 2, and 16 patients, respectively. Of the 18 patients with a rheumatic MV, no, mild and moderate mitral stenosis existed in 14, 3, and 1 patient, respectively. A rheumatic tricuspid valve was not detected in any patient, and rheumatic moderate and severe aortic regurgitation was also found in 1 and 1 patient, respectively. The normal controls were free of abnormalities in the cardiac valves and ventricular systolic function and underwent transesophageal echocardiographic studies to identify cardiac sources of embolic stroke or transient ischemic attack. All patients underwent 2-dimensional Doppler echocardiography and 3D transesophageal echocardiography on the same day.
A 2- to 7-MHz, real-time, 3D transesophageal echocardiographic Xmatrix-array transducer (X7-2t probe, iE33 system, Philips Medical Systems, Andover, Massachusetts) was used to obtain a 3D image. A full volume image was acquired over 4 consecutive heartbeats while the patient held their breath after optimizing the gain, compression controls, and time gain compensation. This process resulted in wide-angled acquisition (65° × 56°, to 102° × 105°).
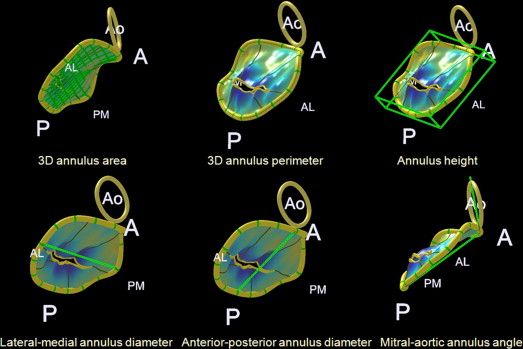
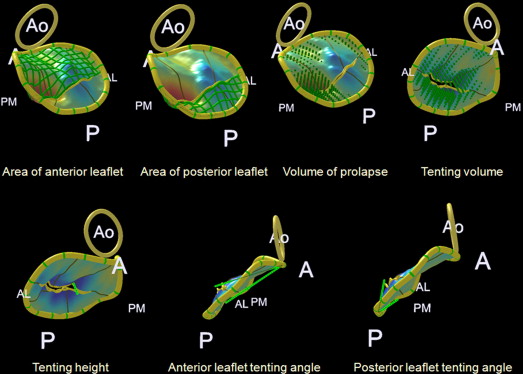
For geometric analysis of the MV leaflet and apparatus, dedicated Q-Laboratory Mitral-Valve-Quantification Software (Philips Medical Systems) was used. The annulus geometry was analyzed by measuring the 3D annulus area and perimeter, annulus height, lateral–medial and anteroposterior annulus diameters, and mitral–aortic annulus angle ( Figure 1 ). The sphericity index of the annulus was defined as the ratio of the anteroposterior to lateral–medial annulus diameters. The leaflet geometry was analyzed by measuring the areas of the anterior and posterior leaflets, total area of the mitral valve leaflet, tenting height and volume, tenting angles of the anterior and posterior leaflets, and prolapse volume ( Figure 2 ). The ratio of the leaflet to annulus areas was defined as the ratio of the total area of the mitral valve leaflet to the 3D annulus area. The ratio of the anterior to posterior leaflets was also calculated. All geometric measurements were performed in a midsystole frame of the 3D volume set.
The left ventricular (LV) end-diastolic and end-systolic and left atrial dimensions were measured with M-mode or 2-dimensional echocardiography. The LV ejection fraction was calculated by measuring the end-diastolic and end-systolic volumes with the modified Simpson method. The MR effective regurgitant orifice area (EROA) was calculated using the proximal isovelocity surface area method.
Statistical analysis was performed with SPSS (SPSS, Chicago, Illinois). The data are reported as the mean ± SD. Comparisons of the mean values among the 3 groups were performed with 1-way analysis of variance and post hoc analysis. Univariate linear regression was performed with EROA as the dependent variable and the other measurements as the independent variables. Multivariate analysis was implemented by stepwise multiple linear regression analysis. The final model, which included variables with p <0.10, was derived to discover independent determinants among parameters with significant univariate correlations (p <0.1). p Values <0.05 were considered statistically significant.
Results
Figure 3 shows representative examples of 3D illustrations of MVs in the normal controls and patients with rheumatic or prolapsed MR. The LV and left atrial dimensions were larger in patients with MR than in the normal controls, with no significant difference between the patients with rheumatic or prolapsed MR ( Table 1 ). The LV ejection fraction was greater in the prolapsed MR group than in the other groups. The patients with rheumatic MR had significantly smaller EROA values than did the patients with prolapsed MR (0.54 ± 0.21 vs 0.82 ± 0.40, p <0.001).
| Parameter | Normal (n = 20) | Rheumatic (n = 18) | Prolapsed (n = 52) | p Value |
|---|---|---|---|---|
| Age (yrs) | 57 ± 13 | 52 ± 17 | 55 ± 14 | 0.486 |
| Body surface area (m 2 ) | 1.71 ± 0.16 | 1.60 ± 0.17 | 1.68 ± 0.17 | 0.105 |
| LV end-diastolic dimension (mm) | 50 ± 4 | 58 ± 6 ∗ | 59 ± 7 ∗ | <0.001 |
| LV end-systolic dimension (mm) | 31 ± 4 | 38 ± 6 ∗ | 37 ± 5 ∗ | <0.001 |
| LV ejection fraction (%) | 62 ± 3 | 61 ± 6 | 65 ± 4 ∗, † | 0.003 |
| Left atrial dimension (mm) | 37 ± 5 | 50 ± 8 ∗ | 46 ± 8 ∗ | <0.001 |
| 3D annulus area (mm 2 ) | 939 ± 145 | 1,353 ± 275 ∗ | 1,334 ± 265 ∗ | <0.001 |
| 3D annulus perimeter (mm) | 112 ± 9 | 133 ± 14 ∗ | 133 ± 13 ∗ | <0.001 |
| Annulus height (mm) | 8.0 ± 1.5 | 7.6 ± 1.4 | 7.9 ± 1.6 | 0.730 |
| Lateral–medial annulus diameter (mm) | 35 ± 2 | 41 ± 5 ∗ | 41 ± 4 ∗ | <0.001 |
| Anteroposterior annulus diameter (mm) | 32 ± 3 | 40 ± 4 ∗ | 38 ± 7 ∗ | <0.001 |
| Mitral–aortic annulus angle (°) | 107 ± 10 | 115 ± 7 ∗ | 112 ± 9 | 0.018 |
| Sphericity index of annulus | 0.89 ± 0.07 | 0.98 ± 0.10 | 0.92 ± 0.14 | 0.061 |
| Area of anterior leaflet (mm 2 ) | 677 ± 122 | 1,039 ± 214 ∗ | 1,000 ± 207 ∗ | <0.001 |
| Area of posterior leaflet (mm 2 ) | 343 ± 53 | 455 ± 119 ∗ | 575 ± 170 ∗, † | <0.001 |
| Total area of mitral valve leaflet (mm 2 ) | 1,019 ± 167 | 1,495 ± 305 ∗ | 1,575 ± 343 ∗ | <0.001 |
| Tenting height (mm) | 4.0 ± 1.2 | 4.8 ± 2.8 | 5.2 ± 2.2 | 0.133 |
| Tenting volume (ml) | 0.8 ± 0.5 | 1.1 ± 1.2 | 1.1 ± 0.9 | 0.390 |
| Anterior leaflet tenting angle (°) | 18 ± 4 | 17 ± 5 | 18 ± 5 | 0.706 |
| Posterior leaflet tenting angle (°) | 37 ± 7 | 35 ± 9 | 33 ± 8 | 0.462 |
| Prolapse volume (ml) | 0.16 ± 0.15 | 0.54 ± 0.63 | 1.06 ± 1.08 ∗ | 0.001 |
| Ratio of leaflet to annulus areas | 1.08 ± 0.02 | 1.11 ± 0.04 | 1.18 ± 0.05 ∗, † | <0.001 |
| Ratio of anterior to posterior leaflet areas | 2.0 ± 0.2 | 2.4 ± 0.5 ∗ | 1.8 ± 0.3 † | <0.001 |
In terms of the MV annulus geometry, the 3D annulus area, 3D annulus perimeter, and lateral–medial and anteroposterior annulus diameters were larger in the MR groups than in the normal controls, with no differences between patients with rheumatic or prolapsed MR. Regarding the MV leaflet geometry, the anterior and posterior leaflet areas and total area of the mitral leaflets in the MR groups were larger than those in the normal controls. No difference was found between the rheumatic and prolapsed MR groups in those parameters, except that the posterior leaflet area was smaller in the rheumatic than in the prolapsed MR group.
The ratio of the leaflet to annulus areas in the prolapsed MR group was higher than those in the rheumatic MR group and normal controls; however, the ratio was not different between the rheumatic MR group and the normal controls. The ratio of the anterior to posterior leaflet areas in the rheumatic MR group was higher than those in the prolapsed MR group and normal controls. Of the patients with prolapsed MR, the ratio of the anterior to posterior leaflet areas was higher in the 18 patients with anterior leaflet prolapse than in the 34 patients with prolapse only in the posterior leaflet (2.0 ± 0.3 vs 1.7 ± 0.3, p <0.001).
To obviate the confounding effect of the location of the prolapsed segments, we selected 16 patients with prolapsed segments in both the anterior and the posterior leaflets from the prolapsed MR group to compare with the other 2 groups ( Table 2 ). The 3D annulus area, 3D annulus perimeter, and lateral–medial annulus diameter were larger in the MR groups than in the normal controls; however, no differences were found between the patients with rheumatic and prolapsed MR. The anteroposterior annulus diameter in the prolapsed MR group was not significantly larger than that in the normal controls. The sphericity index in the rheumatic MR group tended to be larger than that in the prolapsed MR group (p = 0.064); however, the difference was not statistically significant. The areas of the anterior and posterior leaflets and the total area of the mitral leaflets in the MR groups were larger than those in the normal controls, with no difference between the rheumatic and prolapsed MR groups. The ratio of the leaflet to annulus areas in the prolapsed MR group was higher than the ratios in the rheumatic MR group and the normal controls. The ratio of the anterior to posterior leaflet areas in the rheumatic MR group was higher than those in the prolapsed MR group and the normal controls.
| Parameter | Normal (n = 20) | Rheumatic (n = 18) | Both Leaflets Prolapsed (n = 16) | p Value |
|---|---|---|---|---|
| Age (yrs) | 57 ± 13 | 52 ± 17 | 56 ± 15 | 0.493 |
| Body surface area (m 2 ) | 1.71 ± 0.16 | 1.60 ± 0.17 | 1.66 ± 0.14 | 0.104 |
| LV end-diastolic dimension (mm) | 50 ± 4 | 58 ± 6 ∗ | 59 ± 5 ∗ | <0.001 |
| LV end-systolic dimension (mm) | 31 ± 4 | 38 ± 6 ∗ | 36 ± 5 ∗ | <0.001 |
| LV ejection fraction (%) | 62 ± 3 | 61 ± 6 | 64 ± 4 | 0.076 |
| Left atrial dimension (mm) | 37 ± 5 | 50 ± 8 ∗ | 47 ± 8 ∗ | <0.001 |
| 3D annulus area (mm 2 ) | 939 ± 145 | 1,353 ± 275 ∗ | 1,289 ± 197 ∗ | <0.001 |
| 3D annulus perimeter (mm) | 112 ± 9 | 133 ± 14 ∗ | 131 ± 10 ∗ | <0.001 |
| Annulus height (mm) | 8.0 ± 1.5 | 7.6 ± 1.4 | 7.8 ± 1.7 | 0.711 |
| Lateral–medial annulus diameter (mm) | 35 ± 2 | 41 ± 5 ∗ | 41 ± 4 ∗ | <0.001 |
| Anteroposterior annulus diameter (mm) | 32 ± 3 | 40 ± 4 ∗ | 36 ± 9 | <0.001 |
| Mitral–aortic annulus angle (°) | 107 ± 10 | 115 ± 7 ∗ | 109 ± 10 | 0.018 |
| Sphericity index of annulus | 0.89 ± 0.07 | 0.98 ± 0.10 | 0.87 ± 0.22 | 0.053 |
| Area of anterior leaflet (mm 2 ) | 677 ± 122 | 1,039 ± 214 ∗ | 1,002 ± 162 ∗ | <0.001 |
| Area of posterior leaflet (mm 2 ) | 343 ± 53 | 455 ± 119 ∗ | 515 ± 136 ∗ | <0.001 |
| Total area of mitral valve leaflet (mm 2 ) | 1,019 ± 167 | 1,495 ± 305 ∗ | 1,518 ± 261 ∗ | <0.001 |
| Tenting height (mm) | 4.0 ± 1.2 | 4.8 ± 2.8 | 4.2 ± 1.8 | 0.512 |
| Tenting volume (mL) | 0.8 ± 0.5 | 1.1 ± 1.2 | 0.8 ± 0.7 | 0.343 |
| Anterior leaflet tenting angle (°) | 18 ± 4 | 17 ± 5 | 17 ± 5 | 0.597 |
| Posterior leaflet tenting angle (°) | 37 ± 7 | 35 ± 9 | 33 ± 11 | 0.406 |
| Prolapse volume (ml) | 0.16 ± 0.15 | 0.54 ± 0.63 | 1.33 ± 1.34 ∗, † | <0.001 |
| Ratio of leaflet to annulus areas | 1.08 ± 0.02 | 1.11 ± 0.04 | 1.17 ± 0.05 ∗, † | <0.001 |
| Ratio of anterior to posterior leaflet areas | 2.0 ± 0.2 | 2.4 ± 0.5 ∗ | 2.0 ± 0.4 † | 0.003 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


