Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, occurring in 1% to 2% of the general population. Adults with degenerative aortic valve (AV) disease have been shown to have an elliptical shaped AV annulus. The goal of this study was to investigate the shape of the aortic annulus in children with BAV, coarctation of the aorta (CoA) with or without BAV, and normal controls with trileaflet AVs using 3-dimensional echocardiography (3DE). We reviewed echocardiograms of children with isolated BAV (n = 40), CoA (n = 26), and controls (n = 40) that included 3DE of the AV. Eccentricity index (EI) was defined as the ratio between the smaller and larger annular dimension. ΔD was defined as the difference between the larger and smaller annular dimension. Patients with BAV had an eccentric AV annulus compared with controls (BAV EI 0.85 ± 0.05 and control EI 0.96 ± 0.03; p <0.001). Subjects with CoA also had a more eccentric annulus than controls regardless of AV morphology (CoA 0.84 ± 0.06; p <0.001). EI was not associated with somatic growth parameters or gender. Among all patients with BAV, AV dysfunction was associated with fusion of the right and noncoronary (R-N) cusps (p <0.001), but there was no association between valve dysfunction and EI. ΔD was higher in both the BAV and CoA groups compared with the control group (BAV 3.4 ± 1.9 mm, CoA 2.8 ± 1.8 mm, and control 0.6 ± 0.4 mm; p <0.001 each). Although there was no significant correlation of ΔD with age in the control group during childhood, ΔD increased with age in the BAV and CoA groups. In conclusion, children with BAV and/or CoA have an elliptical shaped AV annulus by 3DE, which is independent of age, gender, or body surface area. AV annular eccentricity may lead to inaccurate measurement of AV annular size if measured by 2DE alone. Considering AV annular eccentricity when balloon sizing the annulus before valvuloplasty may help improve interventional results in some patients.
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, occurring in 1% to 2% of the general population. Coarctation of the aorta (CoA) is less common (0.06% to 0.08%), but 50% to 80% of patients with CoA have a BAV. Patients with BAV may present as neonates with critical aortic stenosis as children with variable degrees of valve dysfunction or as adults with early calcific aortic valve (AV) disease. BAV cusp morphology, that is, fusion of the right-noncoronary (R-N) versus right-left coronary (R-L) cusps, may affect the risk of valve dysfunction and need for intervention in children. Previous studies of adults with degenerative AV disease have shown that the AV annulus is elliptical in shape using several 3-dimensional imaging techniques including magnetic resonance imaging, computed tomography, and 3-dimensional echocardiograpy (3DE). However, the shape of the aortic annulus in pediatric patients with BAV and/or CoA has not been studied to date. It was the goal of this study to determine whether the aortic annulus in children with BAV and/or CoA is eccentric compared with children without heart disease and evaluate whether the degree of eccentricity is related to cusp morphology or valve dysfunction.
Methods
We retrospectively analyzed pediatric echocardiograms performed from January 2012 to October 2013 at the Yale New Haven Children’s Hospital and included 3DE views of the AV. We included patients with no significant heart disease (i.e., normal or trivial intracardiac left-right shunt) and trileaflet aortic valves (TAVs; CTL group), BAV (BAV group), and CoA with or without associated BAV (CoA group). We reviewed the medical record of each patient to obtain demographic, diagnostic, treatment, and outcome information.
Nonsedated transthoracic echocardiograms were performed using the Philips iE33 (Philips Medical Systems, Andover, Massachusetts). Probe frequency was selected according to patient size. 2DE was performed and analyzed according to the guidelines of the American Society of Echocardiography. 2DE measurements were performed off-line (Synapse Cardiovascular version 4.0.9; FUJIFILM Medical Systems U.S.A., Inc., Stamford, Connecticut) and Z-scores of aortic structures documented. Parasternal short-axis view was used to determine AV cusp morphology. Fusion of the R-L cusp was defined as the anterior leaflet including the right and left coronary artery origins. Fusion of the R-N cusp was defined as 1 leaflet including the right coronary artery origin and usual position of the noncoronary cusp. For the purpose of this study, we defined valve dysfunction as at least moderate stenosis (mean gradient of at least 20 mm Hg), moderate insufficiency, or a history of aortic balloon valvuloplasty.
Focused 3DE 1-beat acquisitions of the AV were acquired from parasternal long-axis view using the 3D zoom function. 3DE data were analyzed off-line using 3DQ software (QLAB version 8.1.2; Philips Healthcare) in the multiplanar reconstruction mode. Aortic annulus was defined as the most basal hinged point of the aortic leaflets using the parasternal long-axis plane. Using the 3D reconstruction, an image in the parasternal short-axis plane at the level of the annulus with the AV en face was obtained and used for measurement ( Figure 1 ). Aortic annulus dimensions were measured in midsystole using the inner edge to inner edge method in the anterior-to-posterior and medial-to-lateral directions to ensure standardization. Eccentricity index (EI) was calculated by dividing the smaller by the larger dimension. A perfect circle would have an EI = 1, and an EI <1 would indicate the presence of asymmetry ( Figure 1 ). The difference in diameter (ΔD) between the larger and smaller 3DE annular dimensions was calculated as an additional measure of annular eccentricity ( Figure 1 ). Aortic annulus area was measured and indexed to body surface area (BSA).
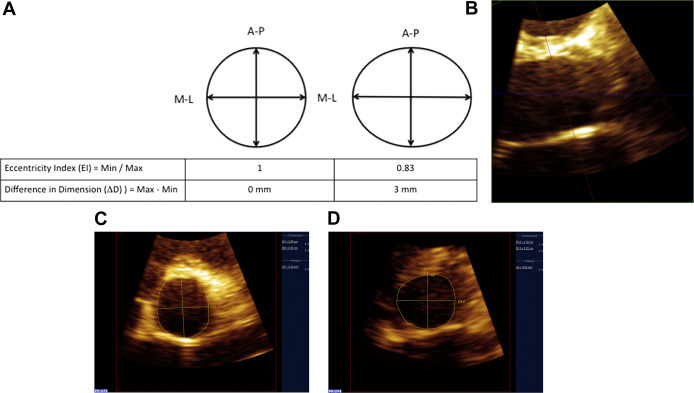
All measurements were performed by a single investigator (CRC). Interobserver variability was assessed for 3DE parameters in 32 patients. The second observer (CGW) was blinded to the measurements of the first observer (CRC). Average measurements of the 2 observers were used in statistical analysis for these patients. The study was approved by the Yale University Institutional Review Board.
For statistical analyses, data are presented as means (SD) and frequencies (percentages) as appropriate. Two-sided student’s t test and Fisher’s exact test were used to compare differences between groups. Multivariate analysis was performed further adjusting for potential confounders. Pearson’s correlation coefficient was used to examine the effect of patient characteristics on variables. To assess interobserver reliability, intraclass correlation coefficient (ICC) for average measurements was performed for 3DE-derived measurements. Statistical analysis was performed using Statistical Package for Social Sciences, version 19 (IBM SPSS, Chicago, Illinois). The set significance level was p <0.05.
Results
A total of 106 echocardiograms were included in the study (median age at the time of the echocardiogram 11, range 0 to 20 years). Comparing groups BAV and CTL, there was no significant difference in age, gender, or BSA ( Table 1 ). Three patients with BAV alone underwent balloon valvuloplasty before study inclusion (all with R-N cusp fusion). Five patients were treated with afterload reducing agents (2 for aortic insufficiency and 3 for a dilated ascending aorta). The BAV group had higher outflow gradients and degrees of insufficiency than the CTL group. The AV annulus and ascending aorta Z-scores were larger in the BAV group compared with the CTL group, but aortic root and sinotubular junction dimension Z-scores were not significantly different. In concordance, the BAV group also had larger 3DE-derived aortic annulus area indexed to BSA. By 3DE, patients with BAV had a more eccentric AV annulus and a larger ΔD compared with the CTL group ( Table 1 , Figure 2 ).
| Variable | Bicuspid Aortic Valve (n=40) | Control (n=40) | p |
|---|---|---|---|
| Male | 29 (73%) | 25 (62.5%) | 0.474 |
| Age [years] | 11.9±5.9 | 10.4±5.7 | 0.248 |
| BSA [m 2 ] | 1.35±0.5 | 1.18±0.4 | 0.129 |
| Mean Aortic Valve gradient [mmHg] | 11±7.8 | 3.5±1.3 | <0.001 |
| ≥ Mild Aortic Insufficiency | 23 (58%) | 0 (0%) | <0.001 |
| Two-dimensional echocardiogram Z-scores | |||
| Aortic Valve Annulus | 0.77±1.1 | 0.04±0.9 | 0.002 |
| Aortic Root | 0.54±1.3 | 0.3±1 | 0.340 |
| Sinotubular Junction | 0.85±1.2 | 0.44±1 | 0.09 |
| Ascending Aorta | 2.2±2.2 | 0.5±1.2 | <0.001 |
| Three-dimensional echocardiogram | |||
| Aortic Valve area / BSA [cm 2 /m 2 ] | 2.7±0.7 | 2.3±0.3 | 0.003 |
| Eccentricity index | 0.85±0.05 | 0.96±0.03 | <0.001 |
| ΔD [mm] | 3.5±1.9 | 0.6±0.4 | <0.001 |
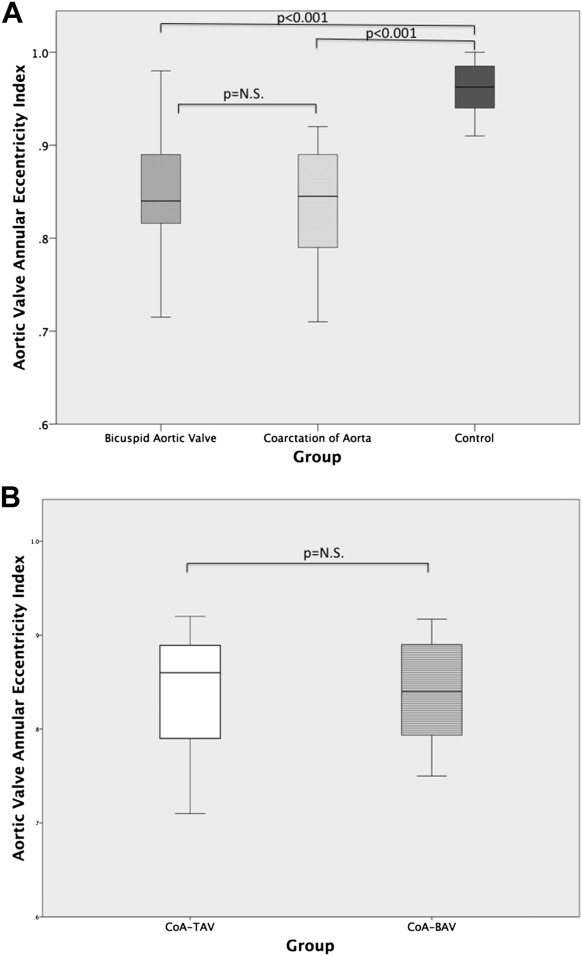
We subsequently compared patients with repaired CoA with the CTL group ( Table 2 ). The CoA group was younger and had a higher percentage of men compared with the CTL group, which was statistically significant. Of all imaging parameters analyzed, only EI and ΔD were significantly abnormal in the CoA group ( Table 2 , Figure 2 ). EI and ΔD remained significantly lower even after correcting for age, gender, and the presence or absence of BAV (p <0.001). Within the CoA group, 11 patients had a BAV (CoA-BAV) and 15 patients had a TAV (CoA-TAV). In a subgroup analysis of patients with CoA-TAV versus CoA-BAV, eccentricity was not significantly different (CoA-TAV EI 0.84 ± 0.07, CoA-BAV EI 0.84 ± 0.06; p = 0.976, Figure 2 ). Thus, even patients with CoA and a TAV have a more eccentric annulus compared with the CTL group.
| Variable | Coarctation of Aorta (n=26) | Control (n=40) | p |
|---|---|---|---|
| Male | 23 (88%) | 25 (62.5%) | 0.02 |
| Age [years] | 7.0±6.9 | 10.4±5.7 | 0.045 |
| BSA [m 2 ] | 0.95±0.7 | 1.2±0.5 | 0.127 |
| Bicuspid Aortic Valve | 15 (58%) | 0 (0%) | <0.001 |
| Mean Aortic Valve gradient [mmHg] | 6.43±6.16 | 3.5±1.3 | 0.027 |
| ≥ Mild Aortic Insufficiency | 1 | 0 | 0.393 |
| Two-dimensional echocardiogram Z-scores | |||
| Aortic Valve Annulus | -0.3±1.6 | 0.04±0.9 | 0.333 |
| Aortic Root | -0.8±1.5 | 0.3±1 | 0.259 |
| Sinotubular Junction | -0.25±1.6 | 0.44±1 | 0.05 |
| Ascending Aorta | 0.04±2.1 | 0.49±1.3 | 0.329 |
| Three-dimensional echocardiogram | |||
| Aortic Valve area / BSA [cm 2 /m 2 ] | 2.4±0.8 | 2.3±0.3 | 0.506 |
| Eccentricity index | 0.84±0.06 | 0.96±0.03 | <0.001 |
| ΔD [mm] | 2.8±1.8 | 0.6±0.4 | <0.001 |
Next, we evaluated the relation between cusp morphology (i.e., fusion of R-N vs R-L phenotype) and EI in all 55 patients with BAV, including CoA-BAV ( Table 3 ). There was no significant difference between gender, age, BSA, aortic 2DE measurements, and indexed AV area between the 2 groups. The EI in R-N compared with R-L morphology was not significantly different. Valve dysfunction was present in 10 of 55 patients with BAV. It was associated with R-N morphology (R-N 9 of 23 [39%] vs R-L 1 of 32 [3%], p = 0.001). However, valve dysfunction was not associated with EI (valve dysfunction EI 0.84 ± 0.04, no valve dysfunction EI 0.85 ± 0.06, p = 0.69).
| Variable | R-L (n=32) | R-N (n=23) | p |
|---|---|---|---|
| Male | 24 (75%) | 19 (83%) | 0.742 |
| Age [years] | 11.4±6.2 | 11.1±6.7 | 0.855 |
| BSA [m 2 ] | 1.3±0.5 | 1.3±0.6 | 0.852 |
| Coarctation of Aorta | 10 (30%) | 5 (22%) | 0.545 |
| Mean Aortic Valve gradient [mmHg] | 7.4±5.3 | 14.2±8.9 | 0.002 |
| ≥ Mild Aortic Insufficiency | 11 (34%) | 15 (65%) | 0.031 |
| Two-dimensional echocardiogram Z-scores | |||
| Aortic Valve Annulus | 0.6±1.4 | 0.5±1.3 | 0.94 |
| Aortic Root | 0.7±1.2 | 0.04±1.4 | 0.082 |
| Sinotubular Junction | 0.7±1.5 | 0.4±1.3 | 0.548 |
| Ascending Aorta | 1.8 ±2.4 | 1.7 ±2.3 | 0.838 |
| Three-dimensional echocardiogram | |||
| Aortic Valve area / BSA [cm 2 /m 2 ] | 2.7±0.8 | 2.5±0.7 | 0.348 |
| Eccentricity index | 0.86±0.05 | 0.83±0.05 | 0.061 |
| ΔD [mm] | 2.1±1.8 | 3.1±2.7 | 0.121 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


