Chapter 12 Thoracic Surgery
In this chapter the care of patients undergoing noncardiac thoracic surgery is discussed. The emphasis is on postoperative care, but preoperative evaluation and intraoperative management are also reviewed. Thoracic surgery patients, particularly those undergoing pulmonary resection or lung volume reduction surgery (LVRS), are commonly elderly, current or former heavy smokers, and highly likely to have other smoking-related diseases. Careful preoperative selection and optimization are essential for good postoperative outcomes.
This chapter is divided into two sections: (1) pulmonary resection; (2) other thoracic surgery. Pulmonary resection is essentially a discussion of the diagnosis, selection, treatment, and postoperative care of patients undergoing surgery for lung cancer. Other topics covered include LVRS, mediastinal tumors, and esophageal resection. Thoracic trauma, massive hemoptysis, and empyema are covered in Chapters 25, 26, and 35.
PULMONARY RESECTION
Surgery for Lung Cancer
Diagnosis
Patients usually have one or more symptoms related to their tumors, such as cough, chest pain, dyspnea, wheeze, or weight loss. Central tumors can obstruct a large airway, causing atelectasis or pneumonia. Other symptoms and signs are suggestive of tumor extension beyond the lung. Pleuritic chest pain may indicate direct tumor invasion of the chest wall. Dysphagia may indicate esophageal involvement. Hoarseness, Horner syndrome, and arm pain are indicative of recurrent laryngeal nerve, sympathetic chain, and brachial plexus involvement, respectively. Lung cancers commonly metastasize to the brain, skeleton, liver, and adrenals. Extrapulmonary or metastatic spread generally precludes surgery. A number of paraneoplastic syndromes are associated with lung cancer (Table 12-1).
Table 12-1 Paraneoplastic Syndromes Associated with Lung Cancer
| Syndrome (hormone secretion) | Clinical Manifestation | Cancer |
|---|---|---|
| Hypercalcemia (parathyroid) | Hypercalcemia, polyuria, hypovolemia, confusion | NSCLC |
| SIADH (ADH) | Hypernatremia | SCLC/NSCLC |
| Cushing syndrome (ACTH, CRH) | Hypertension, fluid retention, weakness, hypokalemia, hyperglycemia | SCLC/Carcinoid |
| Acromegaly (GH, GHRH) | Bony overgrowth | SCLC/Carcinoid |
| Gynecomastia (HCG) | Breast enlargement | SCLC/NSCLC |
| Myositis/myopathy | Proximal weakness, myalgia | SCLC/NSCLC |
| Myasethenic syndrome | Weakness, fatigability | SCLC |
| Brain/spinal cord/peripheral neuropathy | Multiple | SCLC |
ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; CRH, corticotropin releasing hormone; GH, growth hormone; GHRH, growth hormone releasing hormone; HCG, human chorionic gonadotropin; NSCLC, non-small cell lung cancer; SCLC, small cell cancer; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Occasionally, lung cancer is asymptomatic and is an incidental finding on a routine chest radiograph. The presence of a large noncalcified mass (<3 cm in diameter) with spiculated margins is highly suggestive of malignancy. The chest radiograph may show consolidation distal to the mass, mediastinal lymphadenopathy, or pleural effusion. Recently, there has been interest in screening high-risk, asymptomatic patients by means of computed tomography (CT) scanning.1
Surgical Suitability for Resection
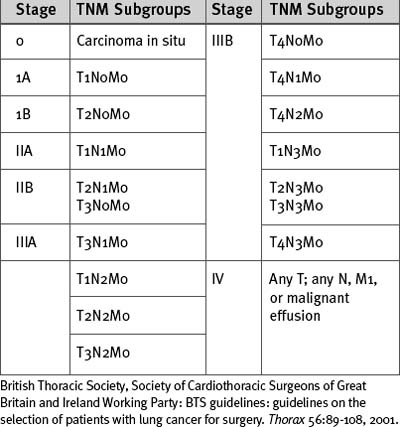
Lung cancer is staged according to the TNM (tumor, node, metastases) classification (Tables 12-2 and 12-3). All patients being considered for surgery should have a CT scan of the chest, liver, and adrenal glands. Percutaneous needle biopsy may be considered for peripheral lesions but is not mandatory. Patients with mediastinal lymph nodes greater than 1 cm diameter on CT scan should undergo a staging biopsy or mediastinoscopy prior to lung resection. The presence of a malignant effusion is a contraindication to surgery, but an effusion due to consolidation distal to an obstructing lesion is not; if there is doubt, a pleural aspirate should be obtained.
Table 12-2 TMN Classification of Lung Cancer
| Primary Tumor (T) | |
| Tx | Primary tumor cannot be assessed or proven |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor <3 cm in greatest dimension, surrounded by lung or visceral pleura, without evidence of invasion more proximal than lobar bronchus |
| T2 | Tumor with any of the following features: >3 cm in greatest dimension involves main bronchus >2 cm distal to the carina invades the visceral pleura associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung |
| T3 | Tumor of any size that directly invades the following: chest wall, diaphragm, mediastinal pleura, parietal pericardium; or tumor in the main bronchus <2 cm distal to the carina but without carinal involvement; or associated atelectasis or obstructive pneumonitis of the whole lung |
| T4 | Tumor of any size that involves any of the following: mediastinum, heart, great vessels, trachea, esophagus, vertebral body, carina; or tumor with a malignant pleural or pericardial effusion or with satellite tumor nodule within the ipsilateral primary tumor lobe of the lung |
| Regional Lymph Nodes (N) | |
| Nx | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes involved by direct extension of primary tumor |
| N2 | Metastases to ipsilateral mediastinal and/or subcarinal nodes |
| N3 | Metastases to contralateral mediastinal, contralateral hilar, ipsilateral, or contralateral scalene or supraclavicular lymph nodes |
| Distant Metastases (M) | |
| Mx | Presence of distant metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases present (includes those in a different lobe of the same lung) |
British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party: BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 56:89-108, 2001.
Fitness for Pulmonary Resection
Curative lung resection involves the removal of a considerable amount of tissue, which results in permanent loss of pulmonary function. Quantitative assessment of pulmonary function is therefore important for stratifying a patient’s surgical risk. However, given that the outcome of nonsurgical treatment of lung cancer is very poor, it may still be appropriate to proceed with pulmonary resection in a patient deemed to be at high risk based on the results of lung function tests.2–5
Routine Assessment
Many patients undergoing pulmonary resection are elderly. Advanced age (<80 years) does not itself preclude pulmonary resection. However, pneumonectomy is associated with an increased risk for mortality in elderly patients, and age should be a factor in deciding suitability for surgery in this patient group.2 The patient’s general clinical state should be evaluated, including changes in exercise capacity or the occurrence of weight loss or anemia. Preoperative weight loss of more than 10%, a body mass index less than 18.5, and a low serum albumin are markers of advanced disease and indicate increased perioperative risk.
All patients should undergo simple spirometry and oximetry measurements prior to pulmonary resection. Some centers also measure the pulmonary diffusing capacity of carbon monoxide (DLCO) in all patients. A forced expiratory volume in the first second (FEV1) that is greater than 1.5 l is considered adequate for lobectomy; an FEV1 greater than 2 l is considered adequate for pneumonectomy. Values less than those are indications for further pulmonary testing. A resting arterial carbon dioxide tension greater than 6 kPa (45 mmHg) also indicates increased perioperative risk.
Pulmonary Assessment of High-Risk Patients
Predicted Postoperative FEV1.
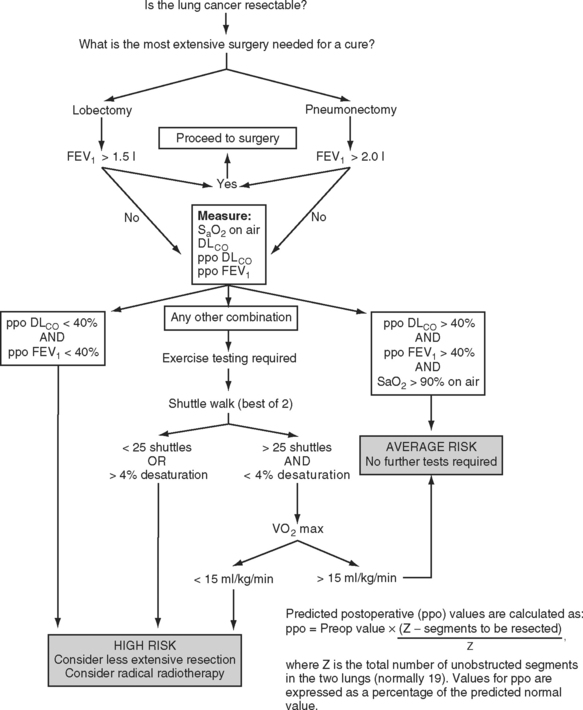
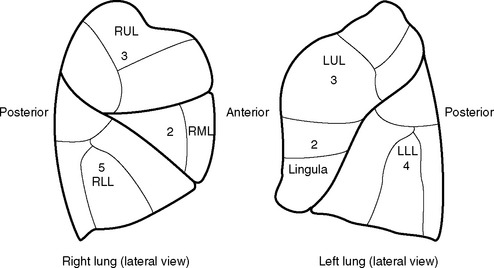
The predicted postoperative FEV1 (ppoFEV1) is calculated from the preoperative FEV1 (preopFEV1) and the proportion of functional lung that is to be removed (Fig. 12-1). The most accurate way to measure the functional contribution of the lung tissue to be resected is by means of split perfusion scanning using technetium (Tc99) macroaggregates or quantitative CT scanning.6 A simple alternative is to estimate the proportion on the basis of the number of anatomic pulmonary segments that are to be removed (Fig. 12-2). To allow safe resection, the predicted postoperative FEV1 should be greater than 40% of normal.
Diffusing Capacity.
The DLCO provides a measure of the severity of parenchymal lung disease—although the measurement probably reflects ventilation-perfusion inequalities rather than impaired function of the alveolar capillary membrane. Reduced DLCO is strongly associated with adverse postoperative outcome. Historically, patients undergoing pulmonary resection with a preoperative DLCO of less than 60% of the predicted value experienced a mortality rate of 25% and a pulmonary morbidity rate of 45%.7 More recent data suggest that if the preoperative DLCO is greater than 45% of the predicted value, acceptable surgical results may be obtained.8
Exercise Testing.
A Vo2 max of less than 15 ml/kg/min indicates a high risk for developing a postoperative cardiorespiratory complication following lung resection.3 A Vo2 max less than 10 ml/kg/min (<40% predicted) indicates that the patient is unsuitable for any form of pulmonary resection, whereas a value greater than 20 ml/kg/min (<75% of predicted) indicates resection up to pneumonectomy is reasonable.3 As with FEV1 and DLCO, a predicted postoperative Vo2 max can also be calculated; a value less than 10 ml/kg/min (<40% predicted) indicates unsuitability for pulmonary resection.9
Integrated Approach to Pulmonary Testing
A number of algorithms have been proposed for pulmonary testing to identify high-risk patients and assess their suitability for surgery. One algorithm, proposed by the British Thoracic Society, is shown in Figure 12-1.2
Preoperative Cardiovascular Evaluation
Clinical predictors of increased perioperative cardiac risk are summarized in Table 12-4. Patients at major risk should have a formal cardiologic assessment, and those with severe coronary artery or valvular lesions should be considered for a revascularization procedure or valve surgery prior to pulmonary resection. Patients at intermediate risk who have good functional capacity do not require further cardiac investigation but, in the presence of poor functional capacity, referral to a cardiologist and cardiac stress testing are warranted. Patients at increased risk for cerebrovascular disease (e.g., a carotid bruit or a history of stroke or transient ischemic attack) should undergo a preoperative carotid Doppler ultrasound study.
Table 12-4 Predictors of Increased Cardiovascular Risk Following Major Noncardiac Surgery
| Major Predictors |
| Unstable coronary syndromes: recent myocardial infarction with evidence of important ischemic risk based on symptoms or noninvasive study; unstable or severe angina |
| Decompensated congestive cardiac failure |
| Significant cardiac arrhythmias: high-grade atrioventricular block; symptomatic ventricular arrhythmias in the presence of underlying heart disease; supraventricular arrhythmias with uncontrolled ventricular rate |
| Severe valvular heart disease |
| Intermediate Predictors |
| Mild angina |
| Previous myocardial infarction based on history of pathologic Q waves |
| Compensated or prior congestive cardiac failure |
| Diabetes |
| Minor Predictors |
| Advanced age |
| Abnormal ECG findings (e.g., left ventricular hypertrophy, left bundle branch block, ST or T wave abnormalities) |
| Rhythm other than sinus rhythm (e.g., atrial fibrillation) |
| History of stroke |
| Poorly controlled hypertension |
Adapted from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542-553, 2002; and from British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party: BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 56:89-108, 2001.
Intraoperative Management
Pulmonary resection for cancer is usually carried out via a lateral thoracotomy incision and involves a lobectomy, bilobectomy, or pneumonectomy. Selective lung ventilation is usually achieved using a double-lumen endotracheal tube. Pneumonectomy is required for a centrally located carcinoma, particularly when the tumor is adherent to hilar structures. Pneumonectomy or bilobectomy is required for tumors that have crossed a lung fissure. Resection of part of the pericardium (intracardiac pneumonectomy) or chest wall may be required for tumor clearance. For tumors involving a bronchus, a “sleeve lobectomy” may be performed; in this surgery a segment of bronchus along with a lobe are excised. The remaining lobes are then reattached to the residual bronchus. Sleeve lobectomy is performed as an alternative to pneumonectomy with the aim of preserving lung function. Bronchotracheal resection with bronchial reattachment is required in cases in which tumors involve the carina. Limited wedge or segmental resection is appropriate for benign tumors, for metastases, and for primary lung cancer in a patient who would not tolerate full lobectomy. However, in patients with primary lung cancer, sublobar resection is associated with increased local recurrence rates compared to lobectomy. Concurrent with pulmonary resection, mediastinal lymph node sampling is usually performed for the purpose of staging the tumor.
Routine Postoperative Care Following Pulmonary Resection
Ideally, patients are extubated following pulmonary resection to minimize air leak and to reduce the tension on bronchial or pulmonary staple lines. However, a period of elective postoperative ventilation is justified for patients with severely impaired gas exchange, hypothermia, acidosis, or ongoing bleeding. Hypothermia significantly increases the incidence of myocardial ischemia; therefore, patients who are hypothermic (<35.5°C) should be warmed to 36.5°C using a forced-air warming blanket.10 Immediately following surgery, a chest radiograph should be obtained to assess lung expansion and the position of drains and to rule out hemothorax. The absence of significant mediastinal shift should be confirmed after a pneumonectomy.
Routine Chest Drain Management
Management of the chest drains differs according to procedure. After lobectomy (and most other thoracic procedures), apical and basal drains are inserted and connected to an underwater seal. Traditionally, these drains have also been connected to low-pressure suction at a level of about −20 cm H2O (approximately 15 mmHg or 2 kPa). More recently, there has been a move away from using suction, with drains just connected to an underwater seal. This strategy can be used in patients with and without air leaks and may lead to a shorter requirement for chest drainage and reduced hospital stay, particularly in patients without air leaks.11 Chest drains are kept in place until drainage is minimal, the residual lung has reexpanded, and any air leak has stopped.