Giant cell and Takaysu’s arteritis
Examples of the various etiologies of thoracic aortic aneurysm disease are listed in Table 11.1. Although the genetics of this disease are multifactorial and not fully elucidated, it is thought that the predominant inheritance of thoracic aortic disease is autosomal dominant with reduced penetrance. Nearly 20% of patients who develop a thoracic aortic aneurysm will have a family history of aortic disease and a familial pattern should be suspected in patients who present at a young age. Younger patients with thoracic aortic dilation should also be evaluated for a bicuspid aortic valve. The presence of a bicuspid valve is highly associated with aneurysm in this population, and these patients may be at higher risk for aortic complications. In older patients, the most common etiology of thoracic aortic aneurysm is degenerative processes associated with increased age; however, hypertension, tobacco abuse, hyperlipidemia, and other genetic factors can result in acceleration of growth. Thoracic aortic aneurysms grow at an average rate of 1–1.5 mm/yr. However, aneurysms that are larger than 5 cm in the ascending aorta and 6 cm in the descending aorta will grow at a faster rate and require more immediate attention.
11.1.2 Clinical Presentation
The vast majority of patients who present with thoracic aortic aneurysms are asymptomatic and the diagnosis is typically made on an imaging test performed for an unrelated indication. Patients may present with chest pain, symptoms of heart failure due to aortic root involvement and subsequent aortic valve insufficiency, local mass effect including compression of trachea or bronchus (wheezing, cough), compression of the esophagus (dysphagia) or hoarseness due to involvement of the recurrent laryngeal nerve. A physical exam may reveal a murmur of aortic valve regurgitation or stenosis, or an early systolic click in the presence of a bicuspid aortic valve.
11.1.3 Evaluation and Diagnostic Studies
Typical chest X-ray findings include a widened mediastinum, deviation of the trachea or displacement of the aortic knob or displaced calcification of the aortic wall. However, none of these findings on chest X-ray are particularly sensitive or specific for thoracic aortic aneurysm. Invasive angiography is the historical gold standard but has largely been replaced by cross-sectional imaging modalities. Table 11.2 highlights the common modalities used to image the thoracic aorta and their respective advantages and disadvantages. Computed tomography (CT) with contrast is typically the most easily accessible and time-efficient modality and is preferred in acute settings (Figure 11.1). Other available modalities for imaging the thoracic aorta include transesophageal and transthoracic echocardiography (Figure 11.2) and magnetic resonance imaging (MRI). Serial imaging utilizing similar imaging modalities is valuable as changes in size over time can dictate the necessity and timing of intervention.
If an aneurysm not requiring surgery is found to involve the aortic root or ascending aorta, then a surface echocardiogram should be performed to evaluate for concomitant aortic valve disease or bicuspid aortic valve disease, which can often be associated with thoracic aortic aneurysms. Once an aneurysm is diagnosed, appropriate surveillance imaging should be performed at 6 months to 1 year for the first year to document aneurysm size stability and then every 1–3 years subsequently depending on the etiology of the aneurysm, the rate of growth and the severity of the concomitant valvular disease.
Table 11.2 Imaging the Thoracic Aorta
| Modality | Advantages | Disadvantages |
| CT angiography | Rapid image acquisition (20–30 s), can use in unstable patients. | Need for iodinated contrast |
| 3D reconstruction allows multiple views/orientations | Radiation exposure (10–20 mSv) – of concern in young patients requiring serial imaging | |
| Ability for post image processing | Image artifacts, particularly in aortic root, may be improved by ECG gating | |
| Aortic size can be overestimated due to oblique cuts through lumen | ||
| MRI/MR angiography | No radiation or iodinated contrast exposure | Caution with use of gadolinium in renal failure |
| 3D, multi-planar and high resolution | Need for breath hold | |
| Dynamic and functional cardiac information available | Time consuming (10–30 min at minimum) depending on center expertise | |
| May be appropriate for serial imaging over many years | Not for use in unstable patients (distance of equipment/staff for resuscitation from patient) | |
| Echocardiography | ||
| Transthoracic | No radiation or iodinated contrast exposure | Cannot visualize entire aorta |
| Can be performed at the bedside, immediate information available | May be limited by technical difficulties, patient body habitus, lung disease | |
| Excellent evaluation of valve function, pericardial effusion and LV function | ||
| Transesophageal | Can visualize aorta from root to GE junction. | Semi-invasive |
| Doppler interrogation of true and false lumen | Requires conscious sedation and patent/secure airway | |
| Evaluation of valvular dysfunction | ||
| Performed at beside |
11.1.4 Treatment
Since most thoracic aortic aneurysms are asymptomatic, the primary indication for treatment is to prevent the development of life-threatening complications, such as dissection or rupture. The likelihood of these complications depends on the size of the aneurysm and its location as well as the skill of the surgeon. In asymptomatic patients, the thresholds for elective surgical intervention are listed in Figure 11.3. In general, non-genetically mediated aneurysms should be considered for surgical intervention when the size is >5.5 cm. In experienced centers, these thresholds might be lower: e.g. when the ascending aorta and arch are ≥5.0–5.5 cm or ≥5.5–6.0 cm in the descending aorta. In patients with the Marfan syndrome and other genetically triggered conditions, the cutoff is lower (4–5 cm) due to their typically younger ages and higher likelihood of developing life-threatening complications over time.
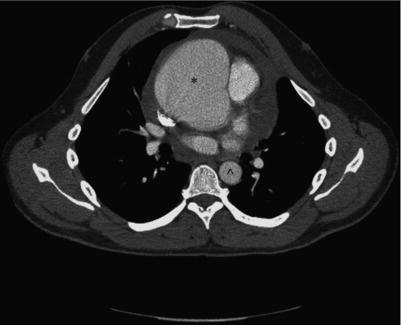
Figure 11.1 CT angiography demonstrating ascending aortic aneurysm. Note size differential between ascending aorta (*) and descending aorta (^).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


