Dynamic blood flow imaging with MRI most commonly employs a PC pulse sequence. Please refer to
Chapter 5 for a more thorough description of the sequence and its use. The terminology used to describe volumetric MRI blood flow imaging can be cumbersome. In this chapter, we will focus on 3D, time-resolved (cine), three-directional PC-MRI. For simplicity, we will call it four-dimensional (
4D)
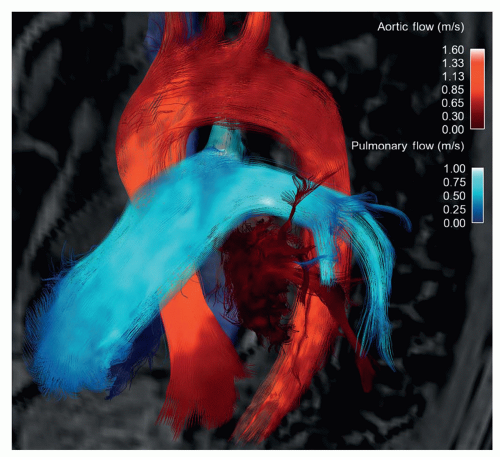
flow, where “4D” refers to 3D plus time, and “flow” to the comprehensive flow field information (mean velocity field and intensity of fluctuating velocity field) obtained by three-directional PC-MRI (
Fig. 33.3). Four-dimensional flow affords an appealing, intuitive visualization of blood flow and unique characterization of vessel hemodynamics not possible with less comprehensive, 2D sequences, or with any other imaging modality.
In conventional 2D cine PC-MRI, the through-plane velocity component of blood flow is measured by applying motion-sensitizing bipolar gradients in this direction. Three-directional VENC can be achieved by applying motion-sensitizing gradients along multiple axes (
27,
28,
29,
30 and
31). As in through-plane PC-MRI, the VENC determines the maximum measurable velocity. However, different VENCs can be used for the different axes, and, at the cost of increased scan time, datasets with multiple VENCs can be acquired. Work in the early 1990s with both nontime-resolved 3D PCMRI (
32) and time-resolved PC-MRI velocity field imaging based on 2D planes stacked to achieve 3D datasets showed the utility of this type of imaging for uncovering complex,
secondary aortic blood flow characteristics such as helices and vortices (
33). Subsequently, true 3D cine PC-MRI or 4D flow acquisitions were developed (
31,
34,
35) and applied to address previously unknown aspects of cardiovascular hemodynamics (
36,
37 and
38).
SCAN TIME AND ACCELERATION
Four-dimensional flow has been limited in the past by long scan times. A large number of k-space lines are needed for three-directional VENC with sufficient spatiotemporal resolution and coverage. Full datasets can take well over 30 minutes to acquire, which is too long for routine clinical medicine. We will discuss the various approaches that are used to reduce scan time so as to make 4D flow clinically feasible. Currently, typical aortic 4D flow protocols require about 10 to 20 minutes of scan time (
Table 33.1).
One commonly used time-saving approach is k-space segmentation, which enables a trade-off between scan time and temporal resolution via the acquisition of multiple k-space lines per cardiac cycle (
39). In addition, substantial advancements have been made to speed up the 4D flow acquisition through k-space undersampling. While undersampling normally causes image artifacts, acquisition, and reconstruction strategies to alleviate, these artifacts exist. Both rectilinear Cartesian and non-Cartesian approaches to k-space sampling can be used.
The Cartesian trajectory is more conventional and widely employed. It is relatively robust to imperfections such as offresonance and eddy currents, but causes fold-over artifacts that can severely degrade the appearance and usefulness of the image when data are not fully sampled. Nevertheless, with the use of multichannel coils, scan acceleration can be effectively employed by undersampling k-space in combination with designated reconstruction algorithms. Parallel imaging methods such as SENSE (SENSitivity Encoding) (
40) and GRAPPA (GeneRalized Autocalibrating Partially Parallel Acquisitions) (
41) reduce scan time by skipping phase-encoding lines during the acquisition, and then restore the missing data in the image or k-space domain. Parallel imaging reduction factors of 2 are routinely used for 4D flow. Higher reduction factors are being explored with new, high channel count receiver coil arrays.
In recent years, several new methods for acceleration of dynamic MRI have been introduced (
42,
43,
44 and
45). Some allow high acceleration factors for applications other than PC, but PC-MRI velocity mapping, unlike many other MRI methods, relies on accurate reconstruction of the phase of the MR signal. This can be a liability when using high acceleration factors and advanced image reconstruction methods. Temporal smoothing introduced at higher k-t acceleration factors, for example, can lead to inaccuracy in flow measurements and particle trace visualizations (
46,
47,
48 and
49). Notably, imaging acceleration based on the theory of compressed sensing has rapidly gained popularity in the past few years (
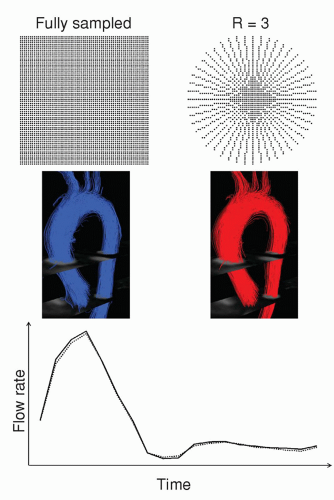
44). Compressed sensing goes a step beyond conventional acceleration methods by exploiting the fact that images have inherent sparsity. Its application in 4D flow is underway and preliminary data show promising results (
Fig. 33.4).
Non-Cartesian k-space trajectories are an alternative approach that limits the severity of undersampling artifacts. For example, a radial acquisition can be employed where k-space is traversed along radial lines through its center, rather than with rectilinear lines (
50). Undersampling in radial acquisitions results in streak artifacts, but this artifact is typically more forgiving, at least visually, than fold-over artifacts. When streaks are acceptable, the radial pattern is faster than a Cartesian approach without sacrificing spatial resolution. In-plane and 3D radial k-space sampling have been successfully applied to reduce the scan time of 4D flow (
51,
52). Another approach is spiral k-space trajectories using oscillating gradient waveforms. This results in reduced scan time by using longer readouts and sampling at a higher bandwidth. Spiral 4D flow can generate data with similar quality and accuracy as that obtained with rectilinear Cartesian sampling, but in 50% to 70% less time (
53). In the near future, further scan time reductions can be expected from combinations of acceleration techniques and non-Cartesian k-space trajectories, as well as from higher acceleration factors made feasible by high channel count receiver coil arrays.
Another time-consuming aspect of aortic 4D flow is respiratory motion compensation. This is most commonly performed using navigator gating. Other approaches include bellows and self-gating. In navigator-gated scans, one-dimensional (1D) images of the lung-liver interface are acquired once per cardiac cycle. In this way, diaphragm motion can be monitored during scanning and used to gate data acquisition with respect to the respiratory cycle. Conventionally, data are acquired during end-expiration. Depending on the subject’s breathing pattern, navigator gating can significantly add to an already long scan time. Several options for increasing the gating efficiency are available. Most sequences use an acceptance window that is dynamically updated based on the subject’s breathing pattern. This can be combined with techniques that take into account the fact that motion occurring during the acquisition of the outer parts of k-space will degrade image quality less than the same motion during acquisition of central k-space. One such method is respiratoryordered phase encoding, which reorders the acquisition of k-space based on the respiratory position (
54). This method has been suggested to reduce motion artifacts in 4D flow and thereby allow the use of a larger gating window (
55). Another option is to use different gating windows for different parts of k-space, that is, narrower window for central k-space (k-space inspired navigator gating [KING], Philips Healthcare, Best, the Netherlands). An alternative to navigators is self-gating, which broadly refers to a sequence that acquires data for motion compensation without the use of navigators. A self-gated 4D flow sequence that repeatedly acquires 1D projections of the entire imaging volume to detect motion has been implemented (
56). To maximize sensitivity for respiratory motion, only the coil element closest to the diaphragm is used and the readout direction is oriented in the feet-to-head direction, similar to conventional respiratory navigators.