Thoracic Aorta
Tim C. Rehders
Hüseyin Ince
Stephan Kische
Christoph A. Nienaber
Endovascular Treatmentof Aortic Dissection
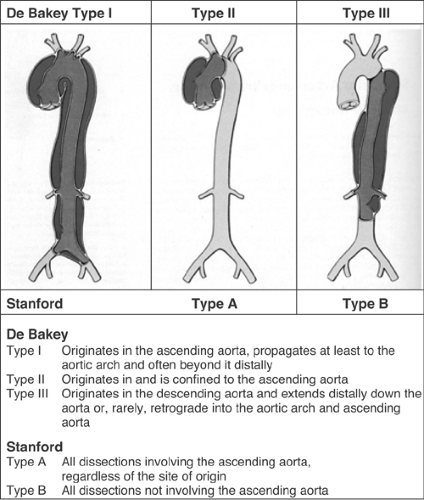
Acute aortic dissection is an uncommon but potentially catastrophic illness that occurs with an incidence of approximately 2.9 per 100,000 per year with at least 7000 cases per year in the United States. Early mortality is as high as 1% per hour if untreated, but survival may be significantly improved by the timely institution of appropriate therapy. Prompt clinical recognition and definitive diagnostic testing are therefore essential in the management of patients with aortic dissection. Conventional treatment of Stanford type A (De Bakey type I and II; see Fig. 9-1) dissection consists of surgical reconstruction of the ascending aorta with complete or partial resection of the dissected aortic segment; thus in type A dissections interventional endovascular strategies have no clinical application except to relieve critical malperfusion prior to surgery of the ascending aorta by distal fenestration in cases of thoracoabdominal extension (De Bakey type I) and peripheral ischemic complications. Conversely, stent-graft placement aims at remodeling of the thoracic descending aorta typically in type B dissection by sealing one (or multiple) proximal entry tears with a Dacron-covered stent, thus initiating thrombosis of the false lumen.1,2,3,4 In addition reconstruction of a collapsed true lumen might result in re-establishment of side-branch flow (Fig. 9-2). Various scenarios of malperfusion syndrome are amenable to endovascular management. These include static or dynamic (by intima invagination) collapse of the aortic true lumen (so called “pseudocoarctation”; Fig. 9-3), static or dynamic occlusion of one or more vital side branches (Fig. 9-4), or enlarging false aneurysm due to patent proximal entry tear.
Although peripheral pulse deficits can be acutely reversed with surgical repair of the dissected thoracic aorta in approximately 90%, patients with mesenteric or renal ischemia do not fare well. Mortality of patients with renal ischemia is 50% to 70% and as high as 87% with mesenteric ischemia.5,6,7 Surgical mortality rates in patients with acute peripheral vascular ischemic complications are similar to those with mesenteric ischemia, reaching an 89% in-hospital mortality rate.8,9,10,11 Operative mortality of surgical fenestration varies from 21% to 61%, which encouraged percutaneous interventional management by endovascular balloon fenestration of a dissecting aortic membrane to treat mesenteric ischemia, a concept discussed as a niche indication in such complicated cases of malperfusion.10,11,12
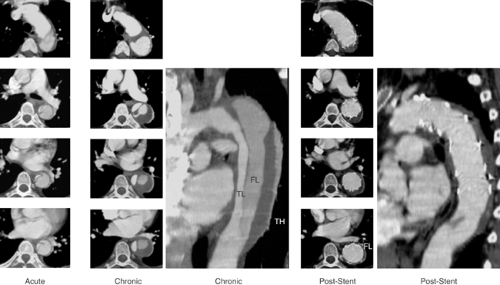
The interventional management of Stanford type B (De Bakey type III) dissection and the use of stent grafts evolved slowly in anticipation of the risk of paraplegia from spinal artery occlusion as seen in up to 18% after open surgery.11,12 With further technical improvement a large series of cases has now been successfully treated in various specialized centers by endovascular stent-graft placement covering entry tears in the descending aorta and even in the aortic arch. Recent studies have demonstrated that closure of proximal entry tears is essential to reconstruct the aortic wall and reduce total aortic diameter. Entry tear closure promotes depressurization of false lumen, thrombus formation in the false lumen (Fig. 9-5), and remodeling of the entire aorta.2,3,12 In the near future combined surgical and interventional procedures even for proximal dissection are likely to evolve.13,14,15
Current Indications for Fenestrationand Endovascular Aortic Repair
The exact role of percutaneous fenestration and stent grafting in the treatment of aortic dissection is not fully established yet. There appears to be a role for interventional concepts in the treatment
of static or dynamic obstruction of aortic branch arteries; static obstruction of a branch can be overcome by placing endovascular stents in the ostium of the compromised side branch, and dynamic obstruction may benefit from stents in the aortic true lumen with or without additional balloon fenestration or side-branch stenting. In classic aortic dissection, successful fenestration leaves true lumen pressure unchanged.16 Sometimes bare stents deployed from the true lumen into side branches are useful to buttress the flap in a stable position.17 In chronic dissection where fenestration of a fibrosed dissecting membrane may result in collapse of the connection between true and false lumen, a stent may be necessary to keep the fenestration open. A rare use of fenestration is to create a re-entry tear for the dead-end false lumen back into the true lumen with the aim to prevent thrombosis of the false lumen and compromise of branches fed exclusively from the false lumen or jointly from the false and true lumen, a concept, however, that lacks clinical proof of benefit. Conversely, fenestration may increase the long-term risk of aortic rupture because a large re-entry tear promotes flow in the false lumen and provides the basis for aneurysmal expansion of the false lumen. There is also a risk of peripheral embolism from a patent but partly thrombosed false lumen.17,18
of static or dynamic obstruction of aortic branch arteries; static obstruction of a branch can be overcome by placing endovascular stents in the ostium of the compromised side branch, and dynamic obstruction may benefit from stents in the aortic true lumen with or without additional balloon fenestration or side-branch stenting. In classic aortic dissection, successful fenestration leaves true lumen pressure unchanged.16 Sometimes bare stents deployed from the true lumen into side branches are useful to buttress the flap in a stable position.17 In chronic dissection where fenestration of a fibrosed dissecting membrane may result in collapse of the connection between true and false lumen, a stent may be necessary to keep the fenestration open. A rare use of fenestration is to create a re-entry tear for the dead-end false lumen back into the true lumen with the aim to prevent thrombosis of the false lumen and compromise of branches fed exclusively from the false lumen or jointly from the false and true lumen, a concept, however, that lacks clinical proof of benefit. Conversely, fenestration may increase the long-term risk of aortic rupture because a large re-entry tear promotes flow in the false lumen and provides the basis for aneurysmal expansion of the false lumen. There is also a risk of peripheral embolism from a patent but partly thrombosed false lumen.17,18
The most effective method to exclude an enlarging and aneurysmal dilated false lumen is the sealing of proximal entry tears with a customized stent graft; the absence of a distal re-entry tear is desirable for optimal results but not a prerequisite. Adjunctive treatment by fenestration and/or ostial bare stents may help establish flow to compromised aortic branches. Compression of the true aortic lumen cranial to the main abdominal branches with distal malperfusion (so called pseudocoarctation) may also
be corrected by stent grafts that enlarge the compressed true lumen and improve distal aortic blood flow.2,3,10,12 Depressurization and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodeling of the entire dissected aorta (Fig. 9-2), and in rare occasions even in retrograde type A dissection.14 Similar to previously accepted indications for surgical intervention in complicated type B dissection, scenarios such as intractable pain with descending dissection, rapidly expanding false lumen diameter, extra-aortic blood collection as a sign of imminent rupture, or distal malperfusion syndrome are accepted indications for emergent stent-graft placement.15,17,18,19 Moreover, late onset of complications such as malperfusion of vital aortic side branches may justify endovascular stent grafting of an occlusive lamella (or fenestration) to improve distal true lumen flow as a first option. Only after an unsuccessful attempt may surgery still be employed, considering that surgical repair failed to prove superior to interventional treatment even in uncomplicated cases; in complicated cases the concept of endoluminal treatment is currently replacing open surgery in advanced aortic centers.1,2,3,17,18,19,20 A summary of treatment options is listed in Table 9-1.
be corrected by stent grafts that enlarge the compressed true lumen and improve distal aortic blood flow.2,3,10,12 Depressurization and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodeling of the entire dissected aorta (Fig. 9-2), and in rare occasions even in retrograde type A dissection.14 Similar to previously accepted indications for surgical intervention in complicated type B dissection, scenarios such as intractable pain with descending dissection, rapidly expanding false lumen diameter, extra-aortic blood collection as a sign of imminent rupture, or distal malperfusion syndrome are accepted indications for emergent stent-graft placement.15,17,18,19 Moreover, late onset of complications such as malperfusion of vital aortic side branches may justify endovascular stent grafting of an occlusive lamella (or fenestration) to improve distal true lumen flow as a first option. Only after an unsuccessful attempt may surgery still be employed, considering that surgical repair failed to prove superior to interventional treatment even in uncomplicated cases; in complicated cases the concept of endoluminal treatment is currently replacing open surgery in advanced aortic centers.1,2,3,17,18,19,20 A summary of treatment options is listed in Table 9-1.
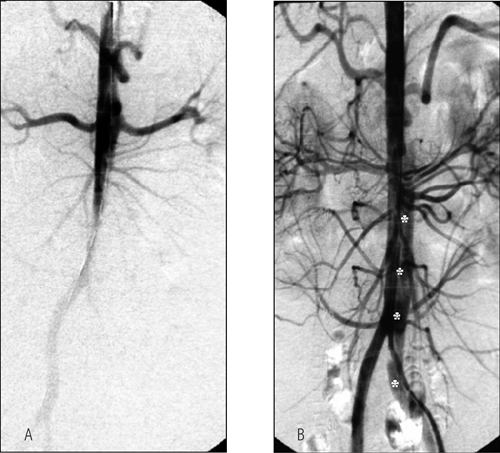
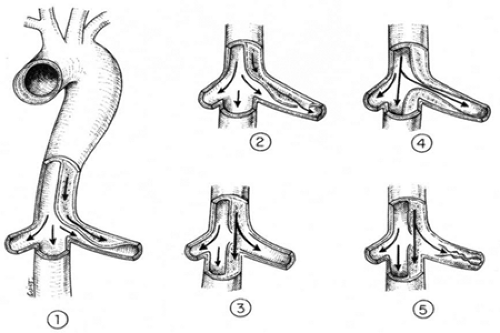 FIGURE 9-4. Possible variants of static or dynamic occlusion of aortic side branches in aortic dissection. |
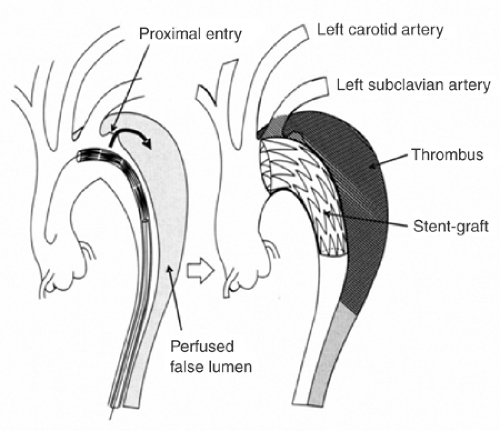 FIGURE 9-5. Concept of interventional reconstruction of the dissected aorta with sealing of the proximal entries, depressurization of the false lumen and initiation of false lumen thrombosis. |
TABLE 9-1. Considerations for Surgical, Medical, and Interventional Therapy in Aortic Pathologies | |
|---|---|
|
Technique of Aortic Stent-Graft Placement
Aortic stent grafts are primarily used to correct compression of the supplying true lumen cranial to major aortic branches and to increase distal flow. Moreover, proximal communications should be sealed to depressurize the false lumen, direct flow to the true lumen, and induce thrombosis in the false lumen with fibrotic transformation and subsequent remodeling of the aortic wall. Stent-graft placement across the origin of the celiac, superior mesenteric, and renal arteries is strongly discouraged for empiric reasons.
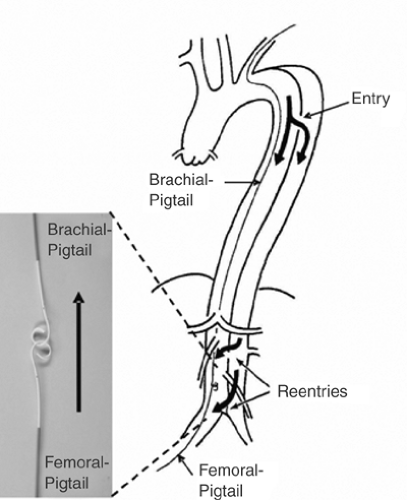
Based on the measurements obtained during angiography, transesophageal echocardiography (mandatory for detection of small entries), contrast-enhanced spiral CT scanning (best technique for unstable patients in an emergency situation), magnetic resonance angiogram (contraindicated for patients with pacemakers or implantable defibrillators), or intravascular ultrasound, customized stent grafts should be used in covering up to 20 cm (and sometimes even more) of dissected aorta and the major tear(s). The procedure is best performed in the catheterization and imaging laboratory using digital angiography and under general anesthesia. The femoral artery is the most popular access site and can usually accommodate a 24F stent-graft system. Using the Seldinger technique a 260-cm stiff wire is placed over a pigtail catheter navigated with a soft wire in the true lumen under both fluoroscopic and transesophageal ultrasound guidance. In complex cases with multiple re-entries in the abdominal aorta, the “embracement technique” with the use of two pigtail catheters is useful (Fig. 9-6). A pigtail catheter that has been installed in the true aortic lumen via the left brachial artery picks up the femoral pigtail catheter in the true lumen of the abdominal aorta and pulls it up into the aortic arch. This procedure ensures definite positioning of the stiff guide wire in the true lumen, which is essential for correct deployment of the stent graft. Carefully advanced over the stiff wire, the launching of the stent-graft is performed with systolic blood pressure briefly lowered to 50 to 60 mm Hg by infusing sodium nitroprusside to prevent dislodgement.21 After deployment, short inflation of a latex balloon may be used to improve apposition of the stent struts to the aortic wall, but only if proximal sealing of thoracic communications is incomplete. Both Doppler ultrasound and contrast fluoroscopy are instrumental for documenting the immediate result or initiating adjunctive maneuvers. For thoracic aortic aneurysm or ulcers, the navigation of wires and instruments is markedly easier, but meticulous imaging using ultrasound and fluoroscopy simultaneously is equally important. A frequent anatomical consideration is the close vicinity between the origin of the left subclavian artery (LSA) and the primary tear in type B dissections. For this reason complete coverage of the ostium to the LSA has to be accepted at times to perform endovascular aortic repair in this aortic pathology adjacent to the LSA. According to observational evidence, prophylactic surgical maneuvers are not imperatively required for safety reasons, but may be relegated to an elective measure after an endovascular aortic intervention when intolerable signs or symptoms of ischemia occur.22 However, prior to intentional LSA occlusion, careful attention has to be paid to potential supra-aortic variants (e.g., presence of a lusorian artery, a nonintact vertebral-basilar system, or vertebral arteries, which originate directly from the aortic arch) and pathologies detected in the course of preinterventional imaging.
Interventional Therapy in an Elective Setting
With the use of bare stents in aortic side branches and sometimes performance of fenestrating maneuvers, compromised flow can be restored in >90% (range 92% to 100%) of vessels obstructed from aortic dissection. The average 30 day mortality rate is 10% (range 0% to 25%) and additional surgical revascularization is rarely needed.23 Most patients remain asymptomatic over a mean follow-up time of about 1 year. Fatalities related to the interventional procedure may occur as a result of nonreversible ischemic complications, progression of the dissection, or complications of additional reconstructive surgical procedures on the thoracic aorta.1,2,3,17,20 Potential problems may arise from unpredictable hemodynamic alterations in the true and false lumen after fenestration and side-branch stenting. These alterations can result in loss of previously well-perfused arteries, or in loss of initially salvaged side branches.
Recent reports suggest that percutaneous stent-graft placement in the dissected aorta is safer and produces better results than surgery for type B dissection. Paraplegia may occur after use of multiple stent grafts but still appears to be a rare phenomenon, especially when the stented segment does not exceed 16 cm. Results of short-term follow-up are excellent with a 1-year survival rate of >90%; tears can be readapted and aortic diameters generally decrease with complete thrombosis of the false lumen. This suggests that stent placement may facilitate healing of the dissection, sometimes of the entire aorta, including abdominal segments (Fig. 9-2). However, late reperfusion of the false lumen has been observed occasionally, underlining the need for stringent MR or CT follow-up imaging. Therefore postinterventional imaging should be done 3 months and 12 months after the procedure, followed by further examinations in yearly intervals. In some patients, follow-up imaging has revealed tears that had initially been overlooked, but required additional stents.
Interventional Therapy in an Emergent Setting
Recently, inclusion criteria for endovascular treatment of acute type B aortic dissection have been reported by Shimono et al.24; in our opinion the following criteria should apply when an emergent stent-graft placement is considered:
Identification of a least one patent primary entry tear in the descending thoracic aorta
Major tear located in the descending aorta proximal to the 10th thoracic vertebra
Absence of severe dilatation (>38 mm in diameter) and/or severe atherosclerotic alterations in the landing zone for stent grafting
Exclusion of severe aortic regurgitation
Exclusion of coronary artery or aortic arch branch ischemia
Femoral and iliac arteries of sufficient size and quality (absence of kinking or significant stenosis) to permit passage of at least a 22F catheter (vessel diameter ≥7.5 mm).
Few cases of emergent stent-graft placement have been so far described. We have recently reported a series of 11 patients treated by emergent endovascular aortic repair of dissection and compared with historic-matched control patients subjected to conventional therapy. All the patients had acute type B aortic dissection complicated by loss of blood into the periaortic space. All procedures were performed successfully with no evidence of periprocedural morbidity, aborted leakage, and ensured reconstruction of the dissected aorta; at a mean follow up of 15 ± 6 months, no deaths were seen in the stent-graft group, whereas four patients had died with conventional treatment. Also, as previously seen in elective endovascular procedures, emergent aortic dissection stent grafting was not associated with excessive peripheral or neurological complications.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree