The Thrombus-Containing Lesion
Emmanouil S. Brilakis
Charanjit S. Rihal
Thrombus-containing lesions are frequently encountered during coronary angiography, especially in patients with acute coronary syndromes; these lesions are associated with an increased risk of periprocedural and long-term complications (1, 2, 3, 4, 5).
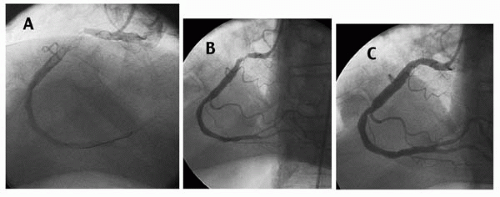
Thrombus most commonly has been defined as an intraluminal filling defect seen in multiple projections similar to the angiographic appearance of deep venous thrombosis (DVT) or pulmonary embolism in other circulations (Fig. 31.1) (1). Compared to angioscopy, coronary angiography has low sensitivity but high specificity for the detection of intracoronary thrombus (6).
Intracoronary thrombus may be present before percutaneous coronary intervention (PCI), or it can develop during or after PCI, in which case it may be more difficult to detect, because post-PCI intraluminal defects or haziness must be differentiated from dissection or tissue protrusion (7).
Early and consistently in the PCI experience, intracoronary thrombus was associated with a significantly higher incidence of serious procedural (procedural failure, in-laboratory abrupt closure, distal embolization, no-reflow phenomenon) and postprocedural (death, myocardial infarction [MI], restenosis) complications (1, 2, 3, 4, 5). Factors associated with higher risk for embolization include a large thrombus burden, mobility of the thrombus (8), and saphenous vein graft (SVG) interventions.
TREATMENTS
The adverse outcomes associated with thrombus-containing lesions have prompted an ongoing search for treatment modalities that aim to:
Dissolve (or stop the expansion of) thrombus, using anticoagulant, antiplatelet, and fibrinolytic (pharmacologic or mechanical) regimens
Remove the thrombus mechanically, using specially designed catheters
Protect the microcirculation mechanically, using proximal or distal protection devices
Dissolution or removal of the thrombus theoretically could prevent distal embolization, allow better visualization of the culprit lesion (which could lead to better choice of treatment), lower the risk of subacute thrombosis, and possibly even lead to better apposition of stents with the vessel wall. For drug-eluting stents (DES), this could enhance drug delivery to the vessel wall.
Primary stenting may be less likely to result in embolization, compared with a strategy of balloon predilatation, presumably because thrombus may become trapped between the stent and the vessel wall, resulting in less distal embolization (9). The implantation of covered stents (either with autologous vein, or with polytetrafluoroethylene [PTFE]) (10,11) could trap the thrombus between the stent graft and the vessel wall, thus reducing the risk of distal embolization.
Treatments That Dissolve Thrombus
Anticoagulation
Systemic anticoagulation (using heparin, low molecular weight heparin [LMWH], or a direct thrombin inhibitor) is used routinely in all coronary interventions and is crucial for patients with thrombus-containing lesions, to prevent extension and allow lysis of the thrombus. Some studies have reported improved outcomes when PCI was done
after prolonged (≥2 days) heparin pretreatment (12), a practice that is currently obsolete. Outcomes after PCI were similar with either heparin or bivalirudin in 567 patients with thrombus-containing lesions in the Hirulog Angioplasty Study (13).
after prolonged (≥2 days) heparin pretreatment (12), a practice that is currently obsolete. Outcomes after PCI were similar with either heparin or bivalirudin in 567 patients with thrombus-containing lesions in the Hirulog Angioplasty Study (13).
Heparin-covered stents may offer resistance to thrombus formation: In a study of 200 patients who received a HEPACOAT stent (Cordis Corporation, Warren, New Jersey) and only aspirin (without thienopyridine) after implantation, only two patients developed stent thrombosis (14). However, heparin-coated stents have not been shown to provide superior outcomes compared to bare metal stents (BMS) (15).
Antiplatelet Agents
Patients with intracoronary thrombus should receive aspirin and a thienopyridine, as is the standard practice for all PCIs. Compared to a loading dose of 300 mg, a loading dose of 600 mg of clopidogrel can achieve platelet inhibition faster, which could be advantageous in thrombotic lesions (16).
Glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors have been shown in several studies to decrease the incidence of postprocedural MI after PCI of the native coronary arteries, but not after PCI of SVGs (17), at the cost of a higher incidence of bleeding. In the c7E3 Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) trial, thrombus resolution occurred in 22% of the placebo and 43% of the abciximab group (p = 0.033) (18), and only patients with increased cardiac troponin T levels had improved outcomes (19). Some investigators advocate the intracoronary administration of GP IIb/IIIa inhibitors, which may result in decreased thrombus burden (20,21).
Pharmacologic Thrombolysis
Thrombolysis can be achieved pharmacologically (through intracoronary or systemic administration of thrombolytics), or mechanically (ultrasound-mediated).
Pharmacologic thrombolysis has not improved clinical outcomes in patients with intracoronary thrombus. In the Thrombolysis and Angioplasty in Unstable Angina (TAUSA) trial, intracoronary urokinase was associated with a higher incidence of abrupt thrombotic closure (15.0% versus 5.9%, p <0.03) (22). Although systemic tissue plasminogen activator (t-PA) was associated with decreased thrombus burden after PCI in patients with UA or non-Q-wave acute MI in the Thrombolysis in Myocardial Infarction IIIA (TIMI IIIA) trial (23), in the TIMI IIIB trial, clinical outcomes were similar in the t-PA and the placebo groups (24). Currently, intracoronary thrombolytic administration is not recommended for unstable angina.
Mechanical Thrombolysis
Ultrasound thrombolysis is a catheter-based technique in which ultrasound delivered at the treatment vessel produces a cavitation effect, resulting in a vortex that pulls thrombus toward the catheter tip, where it is lysed or liquefied to subcapillary size (25). In the Acolysis during Treatment of Lesions Affecting Saphenous Vein Bypass Grafts (ATLAS) trial, 181 patients with an acute coronary syndrome undergoing PCI in SVGs were randomized to ultrasound thrombolysis or abciximab. Compared with abciximab patients (n = 89), ultrasound thrombolysis patients (n = 92) had lower procedural success (63% versus 82%, p = 0.008) and higher incidence of non-Q-wave MI (19.6% versus 7.9%, p = 0.03) (25). Therefore, ultrasound thrombolysis also currently is not recommended in patients with thrombus-containing lesions.
TREATMENTS THAT REMOVE THROMBUS
Thrombus can be removed by using a thrombus aspiration device. Several such devices are currently available.
Export Catheter
The Export catheter is usually used in conjunction with the GuardWire (Medtronic AVE, Santa Roza, California) balloon distal protection system, but it also can be used in isolation (26). It consists of a guidewire lumen and an aspiration lumen, through which suction is applied. In case reports and
case series, use of the Export catheter reduced the thrombus burden in thrombus-containing lesions (26, 27, 28).
case series, use of the Export catheter reduced the thrombus burden in thrombus-containing lesions (26, 27, 28).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree