26 The Thrombus-Containing Lesion
 Introduction
Introduction
Thrombus is a hallmark constituent of active, unstable atherosclerotic plaques commonly found in patients with acute coronary syndromes. Over the past three decades, percutaneous coronary intervention (PCI) has achieved high success rates with the ever-increasing inclusion of complex target lesions.1 However, despite this impressive progress, one critical component, the thrombus, remains a formidable obstacle to revascularization. It continues to constitute a hazardous element whose presence threatens PCI-related major adverse coronary events (MACE), stent thrombosis, increased rate of in-hospital complications, 6-month recurrent MI, and death.2–6 Consequently thrombus exerts a major impact on the performance and outcome of primary and rescue interventions7 (Table 26-1), although the optimal treatment of thrombotic lesions is still enigmatic and controversial. The aim of this chapter is to present the role of thrombus in the pathophysiology of acute coronary syndromes, describe its unique morphology, outline its impact on interventions and outcomes, present available modalities for its identification, delineate pharmacological therapy, and consider the tools and techniques that may be used for thrombus removal. A specific set of challenging thrombus-containing lesions and their management options is highlighted.
TABLE 26-1 The Impact of Thrombus on Percutaneous Coronary Intervention
 Pathophysiology of Thrombus
Pathophysiology of Thrombus
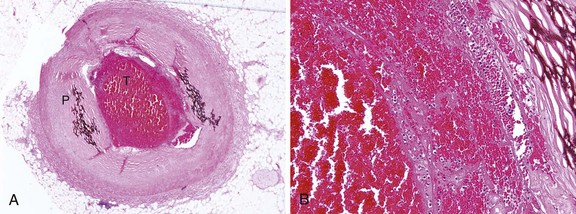
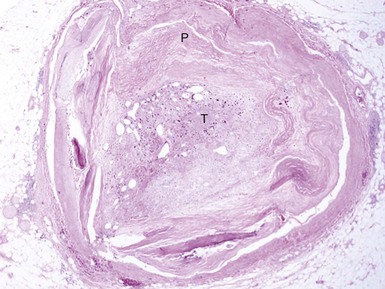
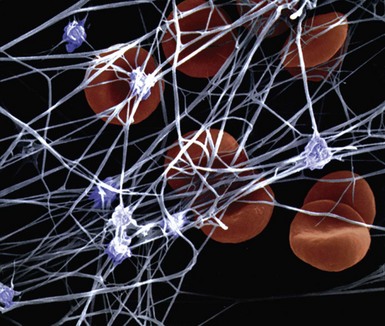
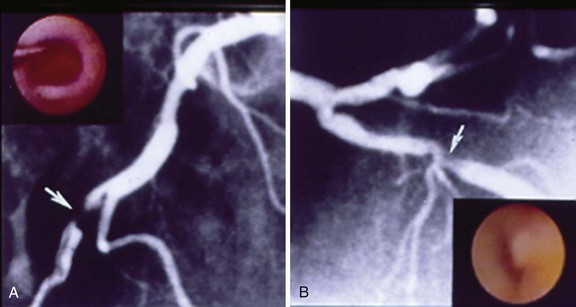
An understanding of the structure of thrombus and its multifaceted characteristics is essential for making proper management choices in the revascularization of atherosclerotic lesions and vessels. A thrombus-containing lesion is formed when the fibrous cap of an atherosclerotic plaque develops structural defects.8 Significant adverse morphological changes in the plaque become manifest by erosion, fissure, or rupture. Such weakening of the plaque’s integrity exposes the inner necrotic core, which enables the transfer of plaque material into the arterial lumen. Cholesterol crystals inside the plaque also perforate the tunica intima, triggering further disruption, with the crystal content identified as an independent predictor of thrombus and clinical events.9 The ensuing contact of exposed, disrupted, and highly thrombogenic subendothelial matrix and plaque with circulating platelets and white blood cells activates the coagulation cascade. The resultant platelet adhesion and aggregation lead to thrombus formation (Fig. 26-1). Furthermore, released tissue factor from the arterial injury directly activates the extrinsic coagulation cascade and promotes fibrin formation. Activated platelets release powerful promoters of vasoconstriction and aggregation, including serotonin, adenosine diphosphate, thromboxane A2, oxygen-derived free radicals, endothelin, and platelet activating factor.10 As the thrombus accumulates to form a critical obstacle (Fig. 26-2), impaired flow dynamics alongside and distal to the thrombotic lesion develop, frequently accompanied by dynamic vasoconstriction and resultant clinical ischemic events.11 During PCI, the notorious instability of thrombus is frequently encountered, as thrombi adhere loosely or at times quite firmly to the underlying plaque and the vessel wall while exhibiting marked friability or rigidity in response to the application of balloons and stents. These seemingly contradictory physical properties stem from the cumulative effect of an assortment of components within the thrombus. Structurally, the thrombus is held by a scaffolding platform of fibrin fibers.12 Two distinct types of branching fibrin fibers are organized in a three-dimensional network. Dense, thin fibers resist deforming mechanical forces and are poorly dissolved by thrombolytic agents. Thick fibrin fibers, on the other hand, are susceptible to the effects of external mechanical forces and readily dissolved by thrombolytic therapy.13 Platelets, red blood cells, vasoconstrictors and procoagulant compounds are anchored to the matrix of the crisscrossing fibers (Fig. 26-3). Abnormalities in platelet function can persist and predict clinical events following PCI.14 In patients presenting with acute coronary syndrome, the clot-adhering platelets typically exert a significantly increased contractile force leading to increased platelet aggregation, so that the entire thrombus exhibits increased elastic modulus.15 The platelets sustain and amplify the coagulant response at the plaque site and release procoagulant platelet-derived microparticles. Various biochemical processes and interactions between activated platelets, red blood cells, fibrinogen, vasoconstrictors, atherosclerotic material, and the vessel wall all have a substantial impact on the fibrin network. These constituents account for the thrombus’s level of activity, stability, or instability and for the overall “aggressiveness” of the thrombus as encountered during intervention. The presence and ratio of the above-mentioned components lead to the formation of distinct thrombus types, each with unique rheolytic and mechanical properties. The two most prominent are the red and white thrombi. They can be detected by angioscopy16 (Fig. 26-4), and their angiographic characteristics correlate with the histology of extracted thrombi.17 The red thrombus has a dense surface with a loose inner core. Transmission or scanning electron microscopy demonstrates loosely packed fibrin and many interspersed red blood cells. The white thrombus consists of a dense structure lacking loose inner spaces, yet it contains a high concentration of platelets with fibrin and only few red blood cells.18 Based on the histopathological analysis of aspirated thrombotic content, erythrocyte-rich (red) thrombus is found in about 35% of patients, predominately in those presenting with low TIMI flow. The platelet-rich (white) thrombus is identified in 65% of cases, especially in the early hours of acute myocardial infarction.19 Noteworthy, however, is the fact that in many patients the occlusive thrombus is a mixture of red and white clot, and the frequent resistance upon extraction attempts suggests that certain clots contain layers upon layers of one thrombus type interspersed with the other.20,21 From a clinical management viewpoint, coronary risk factors such as hypercholesterolemia, smoking, and male gender adversely influence plaque morphology in patients with acute coronary syndromes and are associated with a higher frequency of thrombus.
 Detection of Thrombus-Containing Lesions
Detection of Thrombus-Containing Lesions
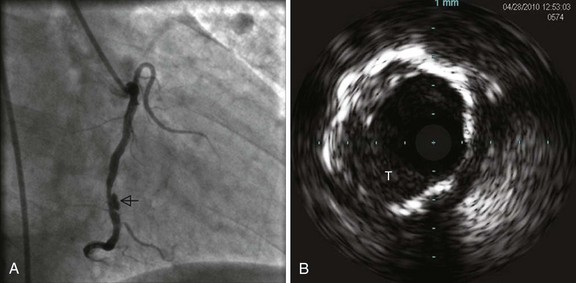
Various imaging modalities are available for the diagnosis of intracoronary thrombus. Numerous studies have demonstrated the poor sensitivity of angiography, although specificity approaches 100% when multiple angiographic views are obtained for verification and strict definitions are used. Nevertheless, angiography remains the practical “gold standard” for the recognition of thrombus, demonstrating the classic findings of reduced contrast density, staining, haziness, irregular lesion contour, “filling defect,” or a smooth convex meniscus at the site of a total thrombotic occlusion. When a thrombus is suspected but not apparent angiographically, the more sensitive techniques for its detection may be utilized, including angioscopy,22 intravascular ultrasound23 (Fig. 26-5), and the recently introduced optical coherence tomography.24
 Thrombus Grading
Thrombus Grading
Grading systems are essential for adequate assessment of the thrombus burden and management decisions prior to and during interventions. The established, most commonly used thrombus grading classification was introduced by the Thrombolysis In Myocardial Infarction (TIMI) Study Group.25 This method is based on the visual angiographic assessment of thrombus size, utilizing a score ranging from grades 0 through 5 (Table 26-2, Fig. 26-6). An important modification to this scoring system5 was recently introduced by the Thoraxcenter investigators, who reclassified the highest grade (i.e., grade 5, which consists of TIMI 0 flow and a strong angiographic suspicion of a large thrombus). When this grade is encountered, either a guidewire or a 1.5-mm balloon (inflated or uninflated) is applied to recanalize the suspected thrombus (Fig. 26-7). As soon as antegrade flow is restored, the exposed, apparently thrombotic content undergoes restratification into a small thrombus burden (grade 1-3) or a large thrombus burden (grade 4) and treatment ensues accordingly. Niccoli and colleagues have introduced a minimalistic thrombus categorization whereby a low grade is assigned to the above-mentioned TIMI grades of 1 to 3 and a high grade corresponding to grades 4 and 5.26 For a more accurate quantitative measurement, Aleong and colleagues have innovatively developed a technique combining edge detection and video densitometry-based quantitative coronary angiography. In early experience with patients exhibiting large intracoronary clots, this modality appeared to quantify the thrombotic volume accurately.27 The advantages and limitations of these thrombus grading classifications are described in Table 26-3. Factors that account for the formation of a specifically high-grade thrombus are depicted in Table 26-4. Among these, the relationship among hyperglycemia, increased white blood cell count, and findings of a high thrombus grade merits special clinical attention.26 These factors also adversely impact the achievement of proper ST-segment resolution during interventions and are associated with a worse PCI outcome.28 A plausible direct relationship between high thrombus content and large vessel size has been known for a long time; however, it has not been uniformly confirmed.26 Intriguingly, despite the convenience of the above-mentioned classifications, most studies of PCI in ischemic coronary syndromes rarely measure, grade, or report the presence of any thrombotic burden.29 This unfortunate omission occurs despite the growing recognition of the structural complexity of thrombi30 and its significant impact on PCI outcome.31,32
TABLE 26-2 Thrombus Grading Scores
* Gibson CM et al. Circulation. 2001;103:2550–2554.
† Sianos G. et al. J Am Coll Cardiol. 2007;50:572–583.
‡ Niccoli G et al. Am J Cardiol. 2010;105:587–591.
TABLE 26-3 Advantages and Limitations of Contemporary Thrombus Grading Classifications
| Advantages |
| Limitations |
2. Underestimation of thrombus presence and size in comparison with angioscopy and intracoronary ultrasound |
TABLE 26-4 Factors Favoring Formation of a High-Grade Thrombus in Atherosclerotic Lesions
| Lesion-related |
| Vessel-related |
Large anatomical size increases thrombus formation and accumulation in native coronary vessels and SVG |
| Myocardial infarction-related |
| PCI-related |
 Limitations of Standard PCI in Thrombus-Containing Lesions
Limitations of Standard PCI in Thrombus-Containing Lesions
In the absence of an apparent thrombus or when only a small thrombus (corresponding to grades 1–2) is detected, the recommended PCI strategy includes standard pharmacotherapy, balloon angioplasty, and stenting.33 Aspiration catheters can be useful as well in this situation. The management of a significant (grade 3) or heavy thrombotic burden (grades 4–5) is considerably more challenging.34 In this scenario, dislodgment of friable thrombotic material by balloon and stent deployment is an issue of significant concern. Embolization of fragmented thrombotic particles obstructs flow within distal arterial segments, side branches, and myocardial microvessels. Overall, distal embolization occurs in the range of 6% to 15% of standard PCI for all lesion types in acute coronary syndromes and is associated with up to a sevenfold increase in the rate of periprocedural MI. In most instances, resultant severe ischemia, microinfarctions, inflammatory response, and contractile dysfunction reduce coronary reserve and cause deleterious effects on recovery and salvage.35 These complications are especially encountered among patients who undergo primary PCI for acute myocardial infarction (AMI).4,5 This was convincingly shown by the TAPAS investigators, who studied the sequelae of angiographically visible distal embolization after PCI in 883 ST-segment elevation MI (STEMI) patients receiving triple antiplatelet therapy.4 Those who sustained angiographically evident embolization had significantly worse outcomes than patients without, as expressed by lower myocardial blush grade, impaired ST-segment resolution, and higher level of myocardial enzyme leakage.4 At 1 year follow-up of these patients, reinfarction occurred in 8.9%, versus 3.0% in those without embolization (P = 0.018). Importantly, the size of the aspirated thrombus was larger in patients with evidence of angiographic embolization versus those without (P = 0.002), and it more often contained erythrocytes (50% vs. 15.7%, P < 0.001, respectively). When compared with the revascularization of thrombus-free lesions, embolization also significantly increases the need for emergency bypass surgery as well as the procedure-related death rate.36 The considerably limited yield of standard PCI in thrombus-containing lesions is further elucidated by the phenomenon of “illusion of reperfusion.”37 Essentially, this term describes a marked discrepancy between primary PCI-gained TIMI 3 flow versus the disappointing, suboptimal degree of myocardial salvage. Notably, the desired grade 3 myocardial blush score is gained in only 28% to 35% of patients in whom the standard PCI induces a TIMI 3 epicardial flow.38 The main cause of this marked discrepancy is the prevalent intracoronary thrombus and related distal embolization, microvessel obstruction, no reflow, and myocardial necrosis. Clearly, patients who develop procedure-related “no reflow” sustain larger-sized infarcts, significantly worse left ventricular (LV) function, and a greater risk of adverse cardiac events and death.39 Thus the thrombus’s deleterious impact as a hazardous material that promotes adverse coronary events and suboptimal outcome should be anticipated and dealt with promptly before, during, and after PCI.32,40 Accordingly, Table 26-5 delineates the indications for a dedicated thrombus removal strategy.
TABLE 26-5 Indications for a Targeted Thrombus Strategy
| Pathology |
| Clinical |
| Targets |
 Pharmacotherapy for Thrombus-Containing Lesions
Pharmacotherapy for Thrombus-Containing Lesions
The mainstay pharmacological treatments for the management of thrombus-containing lesions include aspirin, heparin, glycoprotein IIb/IIIa platelet receptor antagonists, thienopyridines (clopidogrel, prasugrel, ticlopidine), and direct thrombin inhibitors. As for the potential benefit of selective administration of intracoronary thrombolytic agents, further exploration and evidence are warranted. Interestingly, a beneficial decrease of the thrombotic burden can be gained with the mainstay agents even before the initiation of an intervention, with resultant improved PCI results and reduced risk of distal thrombotic embolization. Specifically, chronic aspirin therapy has been shown to decrease the thrombotic burden before stent deployment,26 heparin to reduce fibrin formation and abolish platelet contractile forces,41 and the glycoprotein IIb/IIIa receptor antagonists to dramatically reduce platelet aggregate size as well as to improve thrombolytic access to the platelet-rich clot’s fibrin.42 However, these agents are less effective in an already formed, active, unstable thrombus,43 especially when it is a high-grade thrombus. For example, significant limitations of these useful pharmacological therapies are quite apparent during the revascularization of old saphenous vein grafts with a large thrombotic content.44 Suboptimal PCI results in these thrombotic vessels are manifested by inadequate ST-segment resolution at 60 minutes, limited prevention of distal embolization, and insufficient revascularization. Thus, revascularization of lesions containing a significant clot burden should incorporate a mechanical thrombus removal strategy45 in order to improve PCI outcome.
 Approach to Mechanical Thrombus Removal
Approach to Mechanical Thrombus Removal
Role of Adjunctive Thrombectomy
The recent publication of several landmark studies5,46 has rekindled interest in mechanical thrombus extraction, especially for the management of STEMI. Contemporary mechanical thrombus removal or dissolution devices can be categorized into four main types, according to their activation mode: (1) manual aspiration catheters, (2) power-sourced thrombectomy, (3) ultrasound-induced sonication, and (4) embolic protection. The potential benefits and limitations of mechanical thrombectomy devices are described in Table 26-6. Technically, thrombus removal devices are user-friendly owing to a relatively small size and convenient rapid delivery. Their efficiency contributes to the reduction of procedure-related radiation exposure. A different classification of these devices defines thrombus extraction modalities as either “simple” (i.e., aspiration-based) or “complex” (i.e., mechanically based).47 This categorization is supported by a recent metanalysis of 17 randomized trials on thrombus removal versus standard PCI in 3,909 patients,48 which found that thrombus removal was associated with a significantly greater likelihood of TIMI 3 flow, myocardial blush grade 3, and ST-segment resolution. The investigators concluded that thrombus removal devices appear to improve markers of myocardial perfusion in patients undergoing primary PCI, with no difference in overall 30-day mortality but an increased likelihood of stroke. The clinical benefits of thrombectomy appeared to be influenced by the device type, with a trend toward survival benefit with manual aspiration catheters and worse outcomes with mechanical devices. Facing the large variability of thrombus content and size among patients with acute coronary syndromes, well-trained operators can choose the device according to the angiographic morphological features of the targeted thrombus burden, often achieving excellent results with any of these tools. The last point was recently reemphasized by a large Dutch study of 812 consecutive patients treated with drug-eluting stents for STEMI. The investigators examined the impact of an underlying thrombus size on procedural outcome, demonstrating the direct, proportional effect of the initial and final thrombotic burden on mortality and its role as an independent predictor of postprocedure MACE and stent thrombosis5 (Fig. 26-8). The TAPAS study investigators44 found that thrombus aspiration is applicable in a large majority of patients with STEMI, affording better reperfusion and clinical outcomes than PCI without a mechanical thrombus removal strategy. These findings confirm previous observations from smaller trials and provide credence to the notion that manual aspiration protects the microcirculation during primary PCI. However, it is unclear whether these findings are attributed to an immediate reduction in thrombotic burden, facilitation of direct stenting, or a combination of the two.49
TABLE 26-6 Utility of Thrombectomy Devices