1.
Severe symptoms of HF with dyspnea and/or fatigue at rest or with minimal exertion (NYHA functional class III or IV)
2.
Episodes of fluid retention (pulmonary and/or systemic congestion, peripheral edema) and/or of reduced cardiac output at rest (peripheral hypoperfusion)
3.
Objective evidence of severe cardiac dysfunction, shown by at least one of the following:
(a) A low LVEF (<30 %)
(b) A severe abnormality of cardiac function on Doppler echocardiography with a pseudonormal or restrictive mitral inflow pattern
(c) High LV filling pressures (mean PCWP>16 mmHg and/or mean RAP>12 mmHg by pulmonary artery catheterization)
(d) High BNP or NT-proBNP plasma levels, in the absence of noncardiac causes
4.
Severe impairment of functional capacity shown by one of the following:
(a) Inability to exercise
(b) 6-MWT distance <300 m or less in females and/or patients aged ≥75 years
(c) Peak VO2 < 12–14 mL/kg/min
5.
History of ≥1 HF hospitalization in the past 6 months
6.
Presence of all the previous features despite “attempts to optimize” therapy including diuretics, inhibitors of the renin–angiotensin–aldosterone system, and beta-blockers, unless these are poorly tolerated or contraindicated, and CRT, when indicated
This population represents those who have HF with preserved EF with tenuous fluid balance, cardiac resynchronization therapy (CRT) nonresponders, those who tolerate suboptimal doses of neurohormonal inhibition due to hypotension or cardiorenal syndrome, those requiring oral diuretic dosages greater than 1.5 mg/kg equivalent of furosemide, intravenous diuretics, or addition of thiazides to loop diuretics, and those requiring intravenous inotropes at any point, recurrent hospitalizations for HF, recurrent appropriate implantable cardioverter–defibrillator (ICD) discharges, persistent symptoms with ADLs , and multiple complex comorbidities. Therapeutic options for these patients include further optimization of medical therapy or addition of CRT (which may or may not be effective), high-risk conventional cardiac surgery, cardiac transplantation, bridge to transplant (BTT ) or destination therapy (DT ) ventricular assist device (VAD ), or palliative care.
2.3 The Evolution of Mechanical Circulatory Support
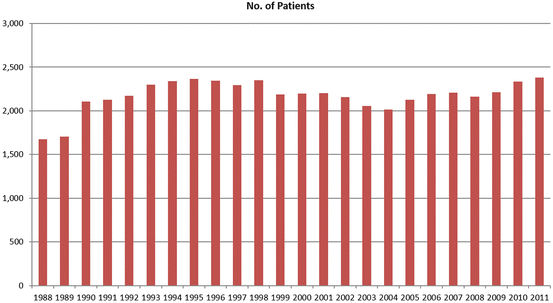
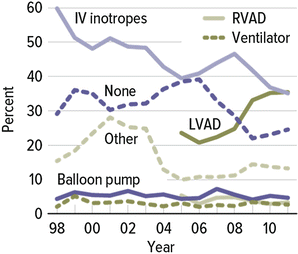
While cardiac transplantation remains the treatment of choice for patients with AHF, application of this procedure has not made a difference epidemiologically in the management of this population. In the USA, there have been no more than 2,000–2,400 transplants (~2.0/100,000 population) performed each year for the last 23 years (Fig. 2.1) due to a severe shortage of acceptable donor organs [6]. However, given the 3.1 million in the USA with HF and low EF, which represents 50 % of overall HF population, 8–10 % of which have AHF and conservatively excluding 75 % for aggressive therapy due to lack of family support, noncompliance, substance abuse, or neurocognitive deficits, the need for advanced heart failure options approaches 30/100,000 population or 93,000, a figure never to be achieved by heart transplantation which currently provides donors to ~1/100,000. In Japan, with an estimated population of HF with low EF slightly over one million, the estimated need using similar assumptions is approximately 18/100,000 population or 23,000! Yet, heart transplantation is performed at a far lower per capita rate and the wait for a suitable donor organ typically exceeds 800 days. Keeping potential recipients alive until a suitable donor is identified is a challenge to say the least worldwide.
Although the first successful VAD was implanted following post-cardiotomy pump failure as a bridge to recovery, the stimulus to develop the field was the inappropriate death rate on the transplant list because of the severity of illness and the long wait until a donor was identified. The advent of left mechanical circulatory support (MCS) with VADs has been a crucial component in improving the lives of those waiting for a transplant and hence the first indication was BTT . Since most will wait from months to several years, MCS must be considered long-term implantation and those not considered transplant candidates may benefit from DT . Initial pulsatile devices, while improving survival and cardiac output, were large and bulky devices with poor durability and a propensity for thrombosis, and as such, had limited application worldwide.
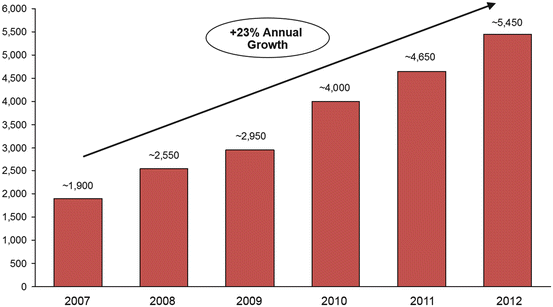
However, in the US REMATCH study, there was a significant and dramatic difference in survival at 24 months between those receiving the HeartMate XVE and those randomized to medical therapy to the point that despite the poor durability of the pump, it was approved by the US FDA for DT (see below). Newer continuous-flow devices have been proven superior to medical care in clinical outcomes and quality of life . As such, following approval of the HeartMate II initially for BTT and now for DT , VADs are being used more and more frequently worldwide [7] (Fig. 2.2).
2.4 LVAD in Practice
2.4.1 Pulsatile Pumps
The first durable LVAD devices were pulsatile pumps such as Novacor (World Heart) and HeartMate XVE (Thoratec). These devices were electrical pumps similar to paracorporeal devices such as the Toyobo Heart, with the advantage of the durable VAD having the pump itself internalized, so patients could be discharged home from the hospital. The HeartMate XVE was approved for both BTT and DT in 2001 and 2003 in the USA, respectively. The HeartMate XVE was a large device comprised of the internal pump and the external controller and batteries. The internal pump, made from titanium predominantly, had a blood chamber, motor chamber, driveline, and inflow and outflow conduits. The pump weighed 1,150 g, and each conduit had a porcine valve within the Dacron graft. The XVE had a maximum stroke volume of 83 mL and could operate up to 120 beats per minute, which could generate flows of up to 10 L/min. The blood and motor chambers were separated by a unique membrane of textured polyurethane, which prevented thrombus formation. The size of this device required a patient to have a body surface area of at least 1.5 m2.
2.4.1.1 Rematch Trial
The randomized evaluation of mechanical assistance for the treatment of congestive heart failure (REMATCH) trial was the pivotal trial in the initial use of VADs, which compared medical therapy to mechanical support in AHF. Patients in this trial were eligible if they were patients with AHF, as defined as a peak oxygen consumption of no more than 14 mL/kg of body weight per minute or continuous need for intravenous inotropic therapy, and ineligible to receive heart transplantation, and were randomized 1:1 XVE or continued medical therapy. The findings of this trial showed that the medical therapy arm had a survival of 25 % at 1 year and an 8 % survival at 2 years. This mortality rate was higher than the rates of breast, lung, and colon cancer and acquired immunodeficiency syndrome [8]. Survival was significantly improved in patients who received a HeartMate XVE with a 1-year survival of 52 % and 2 years of 23 %, with a median survival of 408 days. The most common causes of death in the device group were sepsis at 41 % and device failure of 17 %. These findings led to the HeartMate XVE approval for DT in 2003.
2.4.2 Continuous-Flow Axial Flow Pumps
The next evolution in durable VADs is a significant change from the older model of pulsatile pumps. These newer pumps are continuous-flow pumps which have the advantages of being significantly smaller as well as greater mechanical reliability with no valves or chambers. The most commonly used device worldwide, the HeartMate II , is an axial flow design with a rotor in the blood flow path suspended by bearings. The HeartMate II experience includes over 13,000 implantations. The HeartMate II began development in 1991 [9] with its first human implant in 2000. It has only one moving part, the rotor, which is suspended in the blood flow path by ruby bearings which, with in excess of eight-year follow-up, have yet to show significant bearing wear. Its speed typically ranges from 8,000 to 12,000 rpm, generating from 3 to 10 L/min of blood flow. It received Conformité Européenne Mark in November 2005, following its phase I trial, where patients improved their NYHA class as well as 60 % walked greater than 200 m than baseline [10], and received both BTT approval in 2008 and DT approval in 2010 by the US FDA.
2.4.2.1 Bridge to Transplantation
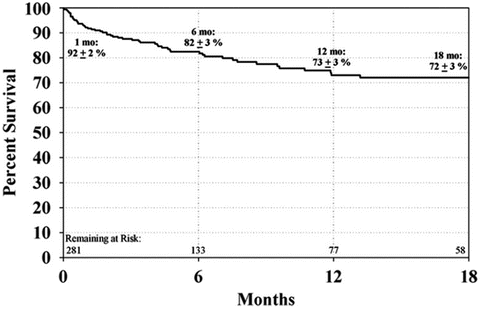
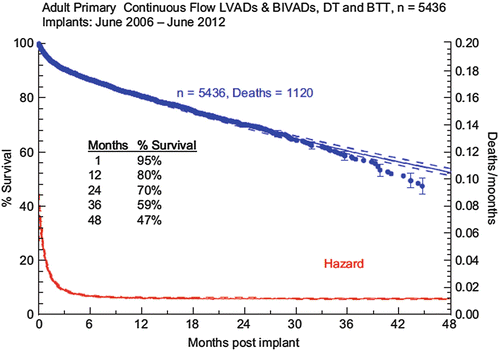
The HeartMate II received BTT approval based on the study published by Miller et al., in which 133 patients were implanted with the HeartMate II and followed them for 180 days [11]. This study found that at 3 months, there was significant improvement in functional status based on NYHA classification (80 % NYHA Class I or II), 6-min walk distance (increase threefold that of CRT), and quality of life based on questionnaires, both Minnesota Living with Heart Failure Questionnaire and Kansas City Cardiomyopathy Questionnaire. The survival rate was 75 % at 6 months and 68 % at 12 months. A follow-up study published by Pagani et al. in 2009 had further survival of 72 % at 18 months, [12] with 79 % either surviving, receiving transplantation, or having recovered and been explanted (Fig. 2.3). There was 92 % survival from any major device malfunction, which was a significant improvement compared to the first-generation pulsatile devices. Most deaths occurred within the first 3 months and were attributable to stroke, infection, or multiorgan failure. Further post-approval data have shown survival at 12 months to be 80 % [13] (Fig. 2.4). These trials and any postanalysis have shown the HeartMate II to be effective at improving patient survival and quality of life while awaiting transplantation. The application of this technology is such that nearly 40 % of all recipients of donor heart receive a BTT VAD before transplant in the USA [6] (Fig. 2.5).
2.4.2.2 Destination Therapy
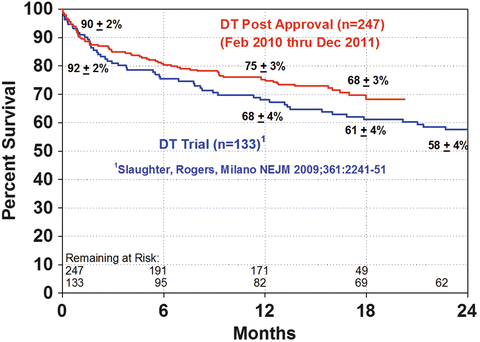
The HeartMate II received DT approval in 2010 in the USA based on the trial published by Slaughter et al. in 2009 [14]. This trial compared the HeartMate XVE to the HeartMate II in patients who were not considered transplant eligible. At the end of 2 years, 46 % of the patients with the HeartMate II had reached the primary endpoint versus 11 % of the patients with the HeartMate XVE. In addition, survival rates at 1 year and 2 years for patients with the HeartMate II were 68 % and 58 %, respectively, compared to 55 % and 24 % with the HeartMate XVE. These were significant advantages for the HeartMate II, which triggered its approval for DT . In post-approval analysis, as a result of improved knowledge of patient care nuances and less moribund recipient selection (see below), there has been improvement in survival of patients with 1-year survival to 75 % and 18-month survival of 68 % [15] (Fig. 2.6). At 2 years, the survival following DT VAD implantation approximates that of heart transplantation at 80 % [16].
2.4.3 Continuous-Flow Centrifugal Pumps
A new design model for continuous-flow LVAD is the centrifugal flow pumps, which also have levitation of the rotor, either through hydrodynamic forces or magnetic forces, or even both. The working idea is that this levitation may cause less wear on the device as well as provide wider gaps in the blood interface leading to less trauma to any blood cells, although explantation of the HeartMate II has shown minimal wear of the device with an expected device longevity of greater than 10 years [17]. The other advantage of these centrifugal pumps is that it can be smaller than the axial flow pumps and can be implanted directly in the pericardial cavity, whereas the HeartMate II implanted in the abdomen. Examples of these devices include the HeartMate III (Thoratec), the DuraHeart LVAS (Terumo), and the HVAD (Heartware). HVAD is the only device to receive formal BTT US approval, although the FDA has persistent unresolved concerns about the higher perioperative neurological events as well as the seven device failures. The HVAD was approved based on the ADVANCE-BTT trial, which compared 140 patients implanted with the HVAD in a study of non-inferiority to 499 control patients who were pooled from the INTERMACS registry after already receiving an FDA-approved VAD , most commonly the HeartMate II. The patients were followed for 180 days. The patient profiles were similar, except INTERMACS score suggested a more advanced population in the control arm, being 3.1 ± 1.3 in the HVAD arm and 2.7 ± 1.3 (p < 0.01) with 44.3 % of the HVAD arm being INTERMACS 3 (stable inotrope dependent) while the control group 51.9 % were profiled as INTERMACS 2 (unstable despite inotropes, vasoactive drugs, or intra-aortic balloon pump) (Table 2.2).
Table 2.2




Intermacs classifications [18]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree