(1)
Anatomy Department, University of Medicine and Pharmacy Carol Davila, Bucharest, Romania
Abstract
Atria develop mostly from the primitive atrium. Initially there is a unique cavity, which has membranous walls (the endocardial tube). During development, these membranous walls will gain muscle fibers.
2.1 The Formation and Septation of Atria
Atria develop mostly from the primitive atrium. Initially there is a unique cavity, which has membranous walls (the endocardial tube). During development, these membranous walls will gain muscle fibers.
The muscle fiber invasion of the atrial wall is discussed later in this chapter.
The inner surface of atria varies during development. The definitive atrial wall contains neighboring structures, therefore we can consider that the atrial wall has an ontogenetic, morphologic and physiologic heterogeneity.
In the adult atrium the auricles develop from the primitive atrium and the definitive atria will integrate parts of the atrioventricular canal (which will later form the vestibular areas), as well as parts from the sinus venosus (which will later form sinus venarum and the dorsosinusal space) and the primitive pulmonary vein (which will form the atrial wall between the ostias of the pulmonary veins).
The dorsal extracardiac mesoderm migrates within the interatrial septum. At the base of this septum, the atrial wall derives from extensions of the endocardial cushions.
Muscle fiber development within the interatrial septum may take place in different ways: through the evolution of adult myocytes from myoblasts, the transformations of the mesenchyme towards the myocyte cell line or the transformation of extracardial mesoderm into myocytes.
Simultaneous development of cardiac myocytes and cardiac vasculature suggests an epicardial involvement in myocyte development (see proepicardial development).
Atrial septation takes place in days 27–36 in the embryos which evolve 4–17 mm. Atrial septation is part of a general and simultaneous process of primitive heart septation. Due to didactic considerations we decided to try to create a schematization according to the dominance and territories of each stage.
2.1.1 The Formation of Septum Intermedium
On the right and left walls of the atrioventricular canal, through cardioglial accumulation, two endocardial prominences develop. After the cardiac tube rotation they take their final position, becoming sagittal – and will be named the superior and inferior endocardial cushions.
Despite their name, the endocardial cushions are genetically lateralized structures.
These cushions will fuse and form the septum intermedium (Figs. 2.1, 2.2, 2.3, 2.4 and 1.14). Both cushions have atrial and ventricular elongations.
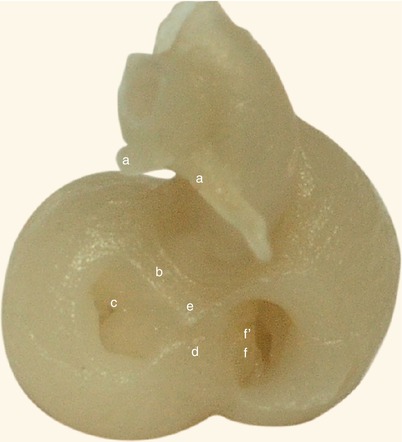
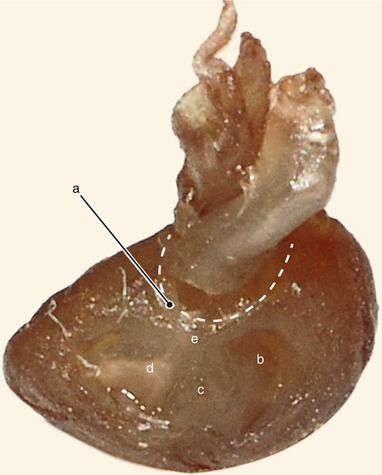

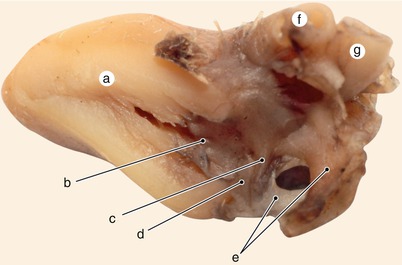

Fig. 2.1
Around 7 weeks old heart, after the removal of the atria. Posterior view. Endocardial cushions are merging. Their extensions are delaminating, leading to cusps formation. The protrusion of the aorta in the superior endocardial cushion separates its mesenchyme into two extensions: a left one, which becomes the mitral-aortic continuity and a right one, which becomes the membranous septum. a Pulmonary arteries, b the future mitral-aortic continuity, c endocardial cushions forming the anterior cusp, d inferior endocardial cushion, e superior endocardial cushion, f, f’ endocardial cushions engaged in forming the septal cusp

Fig. 2.2
Around 7 weeks old heart. After atrial removal, the aorta is also removed at the level of the conotruncal junction. The merging between the wall of the aorta and the septum intermedium is visible. a Aortic protrusion in the superior endocardial cushion, b septal cusp, c inferior endocardial cushion, d anterior mitral cusp, e superior endocardial cusp

Fig. 2.3
The aortic protrusion separates the superior endocardial cushion into a left extension, which will generate the mitral-aortic continuity and a right one, which will generate the membranous atrioventricular septum. Conal septum superior part (arrowhead) separates the aorta from the pulmonary trunk, subvalvulary. a Pulmonary artery, b the left extension of the superior endocardial cushion, c anterior mitral cusp, d inferior endocardial cushion, e the right extension of the superior endocardial cushion, f superior bulboventricular sulcus

Fig. 2.4
A 12 week old heart. Sagittal section, left to the interatrial septum. The way that different structures fuse at the base of the septum is visible. a Left ventricle, b anterior mitral cusp, c superior endocardial cushion extension, d inferior endocardial cushion extension, e fascicular and membranous part of the septum primum, f pulmonary trunk, g aorta
The formation of septum intermedium finalizes in weeks 6–7 with the help of the mesoderm (probably extracardial mesoderm) which migrates at this level through the septum primum (Fig. 2.4).
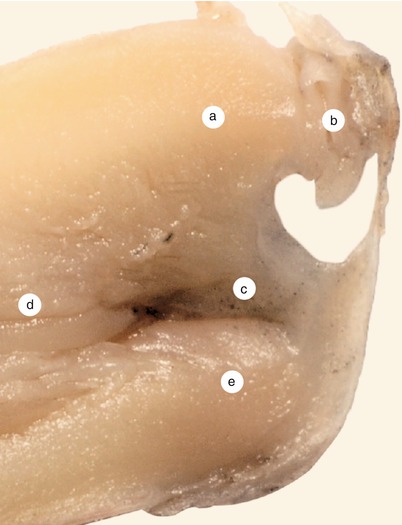
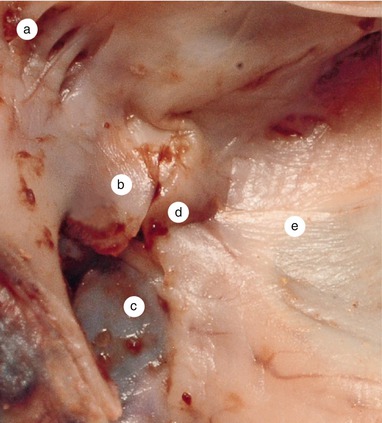
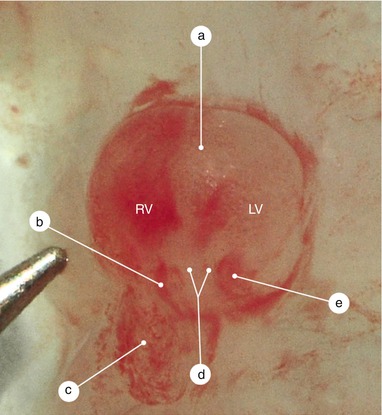

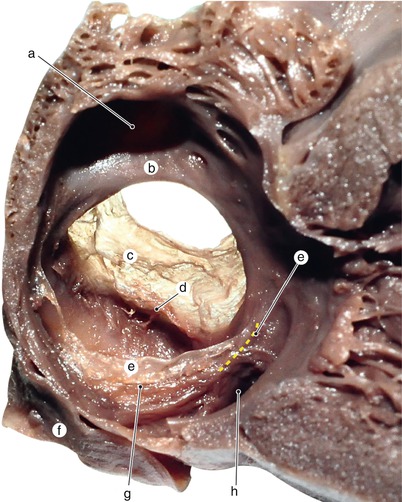
Fig. 2.5
Sagittal section at the right of the previous. The continuity of the interventricular septum, with the endocardial cushions about to merge, is visible. Septum primum material takes part in the fusion of the endocardial cushions in an obvious manner. a Inferior endocardial cushion, b coronary sinus, c septum primum completes the fusion of the two cushions, d interventricular septum, e superior edocardial cushion

Fig. 2.6
A 12 weeks old heart. The left side of the interatrial septum base. A defect in the merging of the endocardial cushions indicates that the structures participate in the forming of the septum intermedium. a Left atrium, b the surface of the superior endocardial cushion, c anterior mitral cusp, d the surface of the inferior endocardial cushion, e the inferior limb of the septum primum

Fig. 2.7
Rat’s heart, 10 days old. The tip of the needle indicates the real size. The heart is reversed. Through the endocardial transparency the septum primum and its two extensions are visible. The right extension merges with the interventricular septum. The atrioventicular ducts are permeable and full with blood. a Interventicular septum, b right atrioventricular duct, c right auricle, d the extension of the septum intermedium which continues the inferior endocardial cushion, e left atrioventricular duct, RV right ventricle, LV left ventricle
The septum intermedium separates right and left atrio-ventricular orifices. These orifices are initially narrow with the aspect of “gullets” (Figs. 2.5, 2.6 and 2.7).
Cells derived from the endocardium classically form the mesenchyme of the endocardial cushions. However, in these cushions also arrive cells derived from the epicardium [1].
2.1.2 The Formation of Septum Primum
A hemodynamic problem has to be solved through atrial septation: how can an orifice temporarily allow blood to flow from the right atrium to the left atrium until birth and this flow stop after birth?
A series of partially unknown events solve this problem, events which we will further describe in detail in the following lines.
An endocardial fold named septum primum develops on the wall of the primitive atrium, opposite to the septum intermedium (posterior-superior wall). This septum primum develops downwards in a falciform pattern. Between septum primum and septum intermedium an orifice is defined – foramen primum. Septum primum continues to develop and fuses with septum intermedium and therefore foramen primum disappears. During this last event another orifice develops in the superior part of septum primum – foramen secundum.
This is the classical succinct way to present the development of septum primum and foramen secundum.
We consider that an alternate theory may be helpful for cardiologists, and we decided to state and describe it.
Septum primum develops on the atrial wall opposite to septum intermedium (postero-superior atrial wall) like an arch-like fascicular mesenchymal mass (a mesenchymal fasciculum which continues the endocardial cushions) which tends to unite both endocardial cushions (Figs. 2.8, 2.9, 2.10, 2.11 and 2.12).
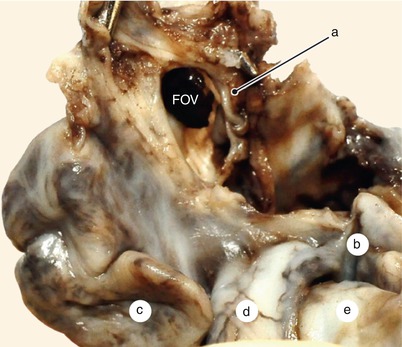
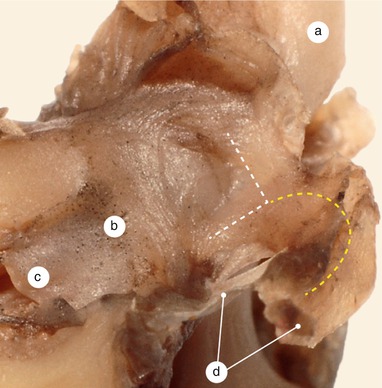
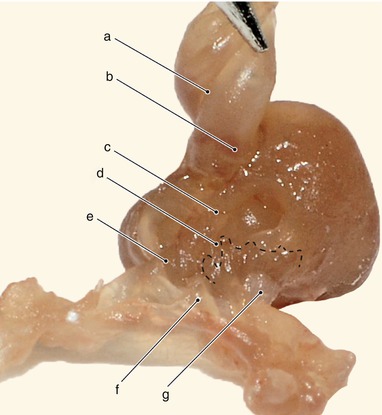
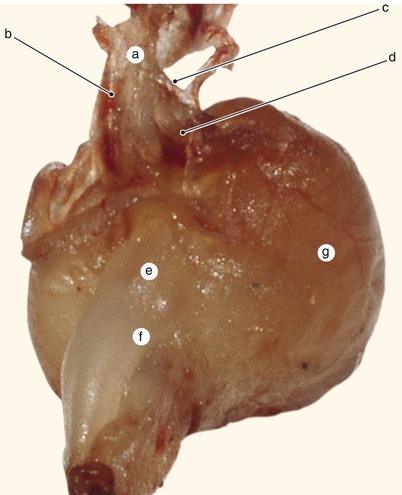
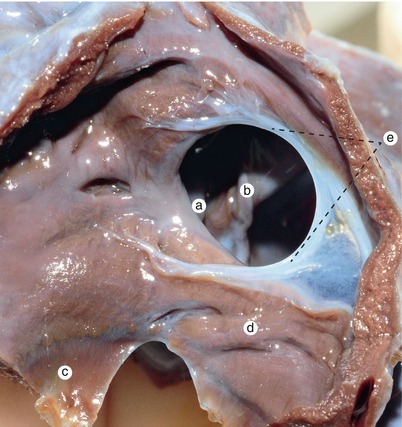

Fig. 2.8
A 12 weeks old heart. The left side of the interatrial septum. The left atrium is removed. The septum primum fascicular part resembles a fold in the atrial celling. a Fascicular part of the septum primum, b left auricle, c right auricle, d aorta, e pulmonary artery, FOV fossa ovalis

Fig. 2.9
A 12 weeks old heart. Left atrium opened, left and posterior view. The fascicular and membranous parts of the septum primum are visible. The origin fascicle of the superior endocardial cushion surface dissociates and participates in the mitral-aortic continuity structure. Between the arms of the “V”, the atrial wall remains membranous for an extended period. Even in adulthood this certain segment can be easily damaged by traction. a Aorta, b mitral-aortic continuity, c anterior mitral cusp, d septum primum fascicular and membranous parts

Fig. 2.10
Approximately 7 weeks old heart. Atria have been previously sectioned at the base and than reversed. The septum primum is visible junctioning with the inferior endocardial cushion. Sinus septi bulges in the right valve of the sinus venosus. The left pulmonary margin flanks the septum primum base. a Spiral septum, b aorta with forming valves, c superior endocardial cushion, d sinus septi, e left pulmonary margin, f septum primum, g right valve. The dotted line shows the inferior contour of the right valve

Fig. 2.11
Approximately 7 weeks old heart. The arterial pedicle is drawn anteriorly. Only the junction between the septum primum and the inferior endocardial cushion remained from the anterior wall. In this area, septum primum is flanked by the two pulmonary ridges (margins). Left to the septum primum, the pulmonary fosset is visible- the place where the primitive pulmonary vein opens. a septum primum, b right pulmonary margin, c left pulmonary margin, d pulmonary vein fosset, e aorta with forming valves, f spiral septum, g left ventricle

Fig. 2.12
Three and a half months old heart. Septum primum viewed from the left atrium. Large interatrial communication, a true “foramen ovale”. The migrating mesenchyme is visible in the thickness of the mitral cups. a Septum secundum, b foramen ovale, c anterior mitral cusp, d inferior endocardial cushion extension, e septum primum with superior and inferior limbs
There is the possibility that this endocardial fold derives from cells from the fusion line of both endocardial tubes. This fusion line is represented by the mesocardic taenia (Fig. 1.8) and corresponds to the base of septum primum.
We should try to understand cardiac septation like a complex and almost simultaneous process which occurs in the atria and ventricles. There are two falciform septums (interatrial and interventricular) which evolve towards each other and will fuse at the level of the fused endocardial cushions. A third septum, the spiral septum, which divides the emerging pole of the ventricle, also fuses with the interventricular septum and septum intermedium, finalizing the septation.
Anteriorly, the septum primum commences from the union of two mesenchymal fascicles, which continue the superior endocardial cushion. The anterior extremity of this septum consequently has the shape of a reversed “V” (Figs. 2.9, 2.18 and 2.22).
Posteriorly, septum primum does not directly reach the inferior endocardial cushion but ends just above it, more precisely, above the pulmonary vein fossa.
The posterior extremity of the septum has a complex aspect. This extremity is flanked laterally by two prominences named the left and right pulmonary ridges (Figs. 2.10 and 2.11) thus named because of their boundary with the pulmonary vein fossa.
The right pulmonary ridge is considered to be Hiss’ spina vestibuli. At its level protrudes into the atrium wall the dorsal extracardiac mesenchyme.
The left pulmonary ridge evolves towards musculization and attenuates at some point due to the evolution of the pulmonary vein towards the left atrium. This ridge also participates in the consolidation of the septum primum base.
The inferior margin of the septum primum contains, throughout its evolution, a sheath of mesodermal cells, endocardially migrated here through the spina vestibuli.
If we closely observe the aspect of the septum primum we can notice the following elements:
a fixed part, basal part – derived from the mesenchyme, continuing the superior endocardial cushion located on the atrial roof. This part quickly suffers a muscular transformation.
a relatively mobile part – the membranous part, with an endocardial fold aspect. Like any other fold it has two sheaths, which cover like a mantle the fascicular part. In our opinion, only this part develops inferiorly and to the left in order to fuse with both endocardial cushions which are on the verge of fusioning (the space between both sheaths represents
A possible way for the extracardiac mesenchyme, which has protruded the septum primum via spina vestibuli, to participate in the endocardial cushions fusion) (Figs. 2.12, 2.13, 2.14, 2.15 and 2.16).
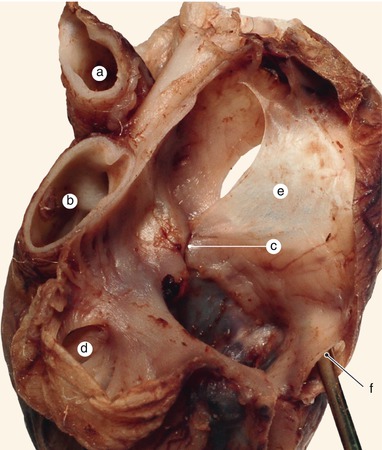
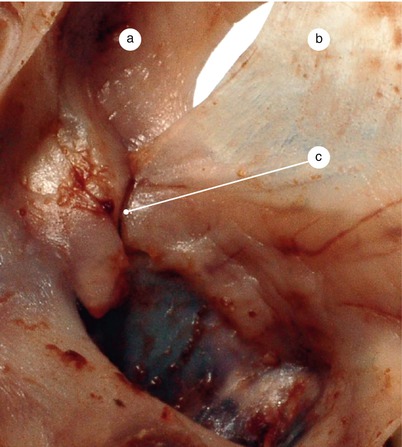
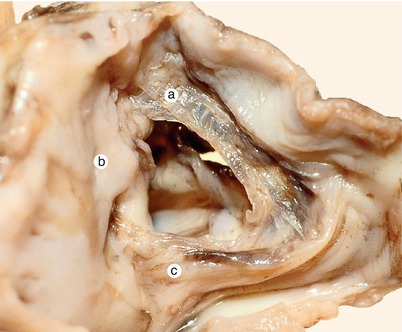
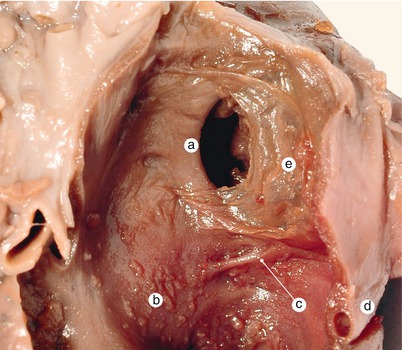

Fig. 2.13
A 4 months old heart. A defect in the merging of the two endocardial cushions material can be seen. Hence delineation of the areas depending on the two cushions and their area of fusion is possible. a Aorta, b pulmonary artery, c a merging vice of the endocardial cushions, d left auricle, e septum primum with superior and inferior limbs, f coronary venous sinus

Fig. 2.14
Detail of the previous image. Septum primum can be clearly seen participating in the merging of the two cushions. a Septum secundum, b septum primum, c merging defect

Fig. 2.15
A 3 months old heart. Inferior limb of the septum primum enters the area of merging of the endocardial cushions. a Superior limb, b merging area, c inferior limb

Fig. 2.16
A 4 months old heart. The left side of the interatrial septum. The inferior cushion extension strengthens the intermediate septum and forming of the anterior mitral cusp. a Septum secundum, b anterior mitral cusp, c inferior cushion extension, d venous sinus, e septum primum
Following the fusion of the three structures (two endocardial cushions and the membranous part of septum primum) and the participation of the extracardiac dorsal mesenchyme, the foramen primum closes (Fig. 2.5).
This evolution might explain why the pathology of septum primum is associated with septum intermedium pathology.
The membranous part of septum primum later suffers a variable fibrous transformation (after the third month) and a much later muscular transformation (just before parturition).
Septum primum evolves like a falciform separating wall. It’s free margin, which is placed anteriorly and concave, will finally become falx septi – the valve of the fossa ovalis. This margin has a superior and an inferior limb (Figs. 2.12, 2.13, 2.14, 2.15, 2.16 and 2.17).
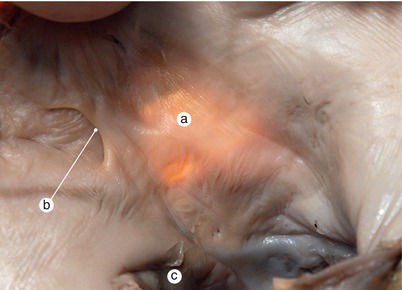

Fig. 2.17
Adult heart. Interatrial septum viewed from the left atrium. Transillumination. The valve of fossa ovale – falx septi- has merged with the septum secundum, closing the interatrial communication. Fossa ovale does not overlap the merging area. a Fossa ovale visible by means of transillumination, b Falx septi, c mitral orifice
The base of septum primum (the part adherent to the wall) develops anteriorly, and passes along with the inferior limb over the endocardial cushions’ fusion line at the level of septum intermedium (Fig. 2.18). In this the way the extracardiac mesenchyme, migrated between the two sheaths of the septum, may participate in the endocardial cushions’ fusion and in the finalization of the septum intermedium. Through the same pathway the extracardiac mesenchyme might reach the anterior mitral cusp (Figs. 2.12 and 2.14).
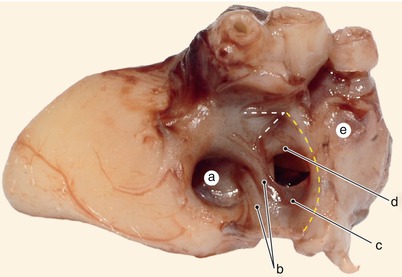

Fig. 2.18
A 3 months old heart. Left side of the interatrial septum. The extensions of the endocardial cushions are very well represented. a Anterior mitral cusp, b endocardial cushions extensions, c septum primum, d septum secundum, e superior vena cava
As we have shown, the membranous part of septum primum has a free margin (anterior – superior) which does not participate in the cushions’ fusion. It has a falciform shape and is shifted towards the left. Between it’s concavity, the anterior atrial wall and septum secundum, it remains throughout fetal development a hemodynamically efficient orifice which allows blood-flow from the right atrium to the left atrium (see preceding fig.). This margin is called falx septi.
The membranous part of the septum primum will finally form the floor of the fossa ovalis. The membranous aspect disappears due to a variable evolution, either through fibrous transformation or muscular transformation.
The space between falx septi and the anterior atrial wall ensures an efficient interatrial communication and is frequently mistaken with foramen secundum. This space, however, is the equivalent of the oval foramen (an ambiguous term to be explained in the following lines).
We consider that the evolution of foramen secundum is not fully clarified in specialty literature.
If foramen secundum would be a compulsory step during atrial septation, it would mean that it appears as a fusion of small orifices which occur in the posterior-superior part of septum primum (according to literature). These small orifices would fuse, resulting a single hemodynamically efficient orifice – foramen secundum. All these events should take place along with the closing of foramen primum – a risky period for the survival of the embryo (if these stages are not well-synchronized there is the risk of right-left atrial blood-flow blockage). Later, through apoptotic processes, the septum primum, which lies anterior to foramen secundum, should disappear and the margin of foramen secundum would become falx septi.
Therefore, we consider that Odger’s [2] statement that says “foramen secundum is rather a lack of development than an involution of an already formed septum” should be reconsidered. Furthermore, we consider that foramen secundum is “an incident” in the evolution of septum primum, which sometimes cannot be compensated and remains after birth as a foramen secundum septal defect (Figs. 2.19 and 2.20).
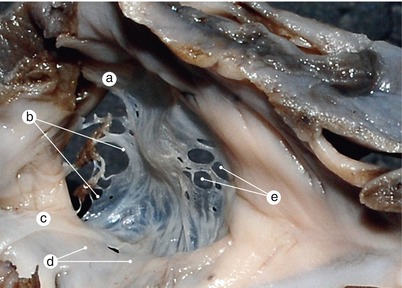
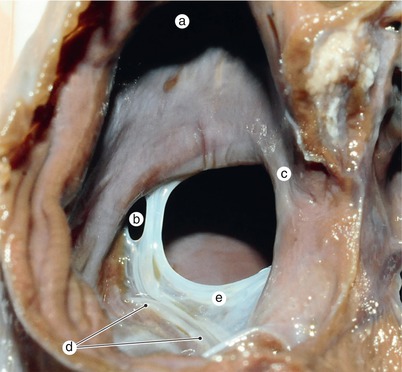

Fig. 2.19
A 7 months old heart. “Classic” ostium secundum in a complex malformation. View from the left atrium. a Superior limb, b Falx septi, c inferior limb of septum primum, d the area of the former ostium primum, e ostium secundum

Fig. 2.20
View from the right atrium. A 5 months old heart. Uncompensated ostium secundum. Muscular septum secundum is very well defined. Between the left valve and septum primum is a fine layer of muscular tissue, perhaps from the protusion of the extracardiac mesenchyme (see also Fig. 2.32). a Superior vena cava, b ostium secundum, c primitive sinus left valve merged with septum primum, d septum secundum, e septum primum
The occurrence of this “incident” is favored by the fact that septum primum does not directly fuse posteriorly with the inferior endocardial cushion due to the interposition of the pulmonary edges and the orifice of the primitive pulmonary vein. In this region, the finalizing of the inter-atrial septum is achieved mainly by overlapping of the left valve of the sinus venosus with the mesenchymal part of the spina vestibuli and the septum primum (Fig. 2.32).
As we underlined, there is a complex aspect of the junction between the septum primum and the inferior endocardial cushion. The superior part of the septum primum and the pulmonary edges limit superiorly the pulmonary vein fossa. Inferior to this fossa there lies the inferior endocardial cushion.
The fusion between the left valve of the sinus venosus and the right pulmonary edge (spina vestibuli) creates along with the septum primum the left wall of a conduit which allows blood to flow from the right atrium to the left atrium. The right wall of this conduit is represented by the right valve of the sinus venosus (Fig. 2.21).
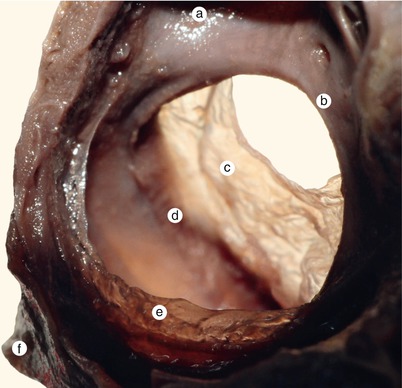

Fig. 2.21
View from the right atrium. The left valve of the sinus venous moulds the posterior edge of a very well defined septum secundum, and than merges with septum primum. The ensemble this way formed with the right valve generates an interatrial conduit. Septum secundum is a true “crista dividens” for the bloodstream (see also Fig. 2.34). a Superior vena cava, b septum secundum, c septum primum, d left valve, e right valve, f inferior vena cava
In order to avoid any confusion this intraatrial conduit is different from the intraseptal conduit, which is going to be detailed in the following lines.
This ensemble creates pressure, which can determine the evolution of the primitive pulmonary vein towards the left atrium. The inclusion of the pulmonary vein within the left atrium wall is guided superiorly by a prominence.
It looks like an endocardial fold and is represented by a posterior elongation of the inferior endocardial cushion (Figs. 2.22 and 2.23). This elongation (septum intermedius) may participate in the fusion of the endocardial cushions. In the same time, this elongation serves as a pathway through which cells of the endocardial cushion participate in forming of the anterior mitral cusp (Fig. 2.24). The opposite edge of this elongation disappears in the posterior left atrial wall. In most cases this ridge involves therefore it strengthens the left side of septum primum’s base. As we underlined before, we consider the elongation of the inferior endocardial cushion is involved in the directing of the incorporation of the pulmonary vein in the left atrial wall (see the following lines).
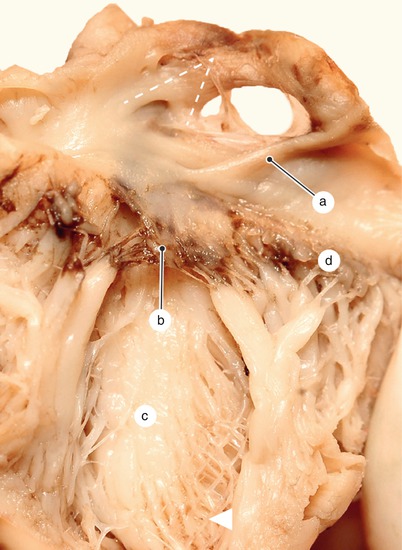
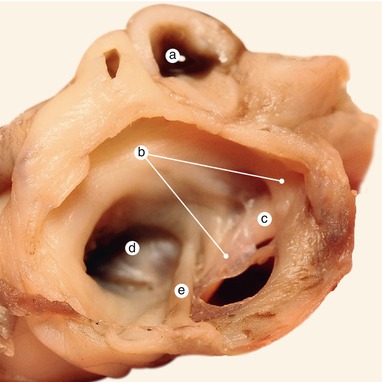
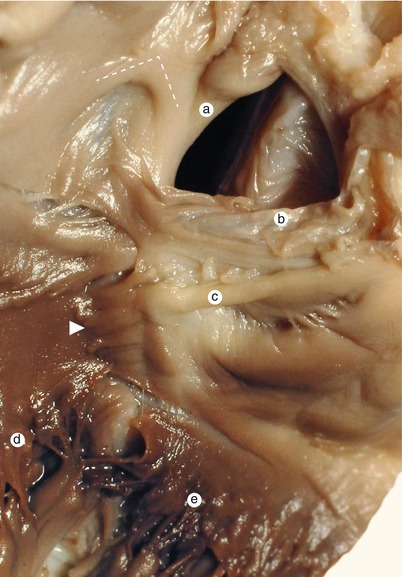

Fig. 2.22
Two and a half months old heart, opened on the obtuse edge. The left side of the septal structures. The form of a reversed “V” of the fascicular part of the septum primum is easily visible, as well as the extension of the inferior endocardial cushion, which forms the anterior mitral cusp. The arrowhead represents Purkinje network. a The extension of the inferior endocardial cushion, b the anterior mitral cusp, c left ventricle, d the posterior mitral cusp

Fig. 2.23
View from the left atrium and posterior of the interatrial septum. Septum primum and septum secundum diverge towards anterior and converge toward posterior. This disposition lessens the interatrial communication. a Pulmonary artery, b septum primum, c septum secundum, d anterior mitral cusp, e extension of the inferior endocardial cushion

Fig. 2.24
The base of the interatrial septum seen from the left atrium. The septum primum origin in reversed “V”, traces on the wall of the primitive atrium an unmusculed, membranous area, of the anterior atrial wall. It is now understood why this area of the atrial wall has a low level of resistance if tractioned. A migrating cellular population enters the mitral anterior cusp (arrowhead) trough the inferior endocardial cushion. a Septum secundum, b septum primum, c extension of the inferior endocardial cushion, d anterior mitral cusp, e posterior cusp
2.1.3 The Formation of Septum Secundum
To the right of the septum primum, on the roof of the right atrium, there is an endocardial fold named septum secundum. This septum has a falciform aspect (it is posterior-inferiorly concave) and has two limbs: superior and inferior (Figs. 2.25, 2.26, 2.27, 2.28 and 2.29).
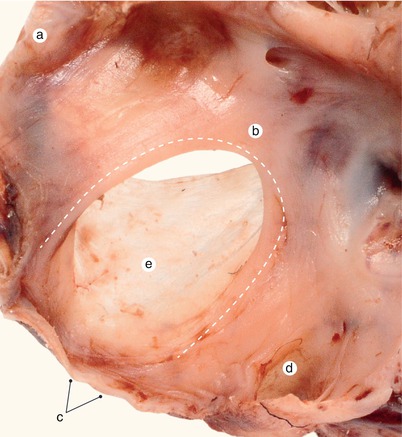
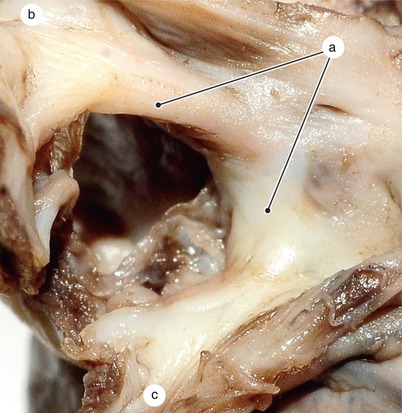
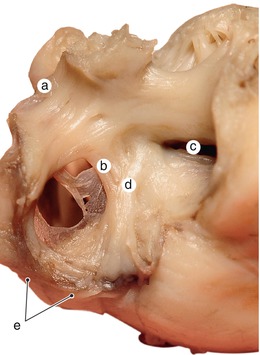
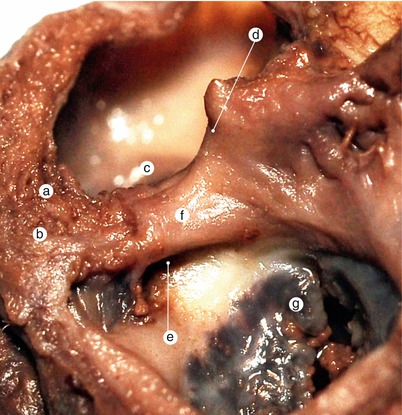
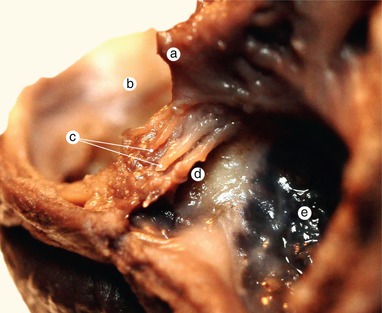

Fig. 2.25
View from the right atrium of the interatrial septum. A 5 months old heart. Septum secundum is well formed, resembling a “tight” fold. a Superior vena cava, b septum secundum, c inferior vena cava, d ostium of the coronary sinus, e septum primum

Fig. 2.26
A 3 months old heart. View from the right atrium. Septum secundum is well defined, resembling a “wide” fold. a Septum secundum, b superior vena cava, c inferior vena cava

Fig. 2.27
Two months and a half old heart. View from the right atrium. Septum secundum towards the anterior has the obvious aspect of a muscular septum and not one of a fold on the wall. a Superior vena cava, b septum secundum, c tricuspid hole, d tendon of Todaro, e inferior vena cava

Fig. 2.28
Postero-superior view of interatrial septum base, after removal of the atria. A 5 months old heart. Notice the dissociation from the base of the interatrial septum. Two mesenchymal axes enter the septal base. The most obvious one (the right one) arises from the sinus septi thickness, then enters the septum secundum base. Its origin may lay in the extracardial mesenchyme of the dorsal mesocaridum, sequestered in the sinus septi mass. This axis will become the Todaro tendon. The less proeminent axis, situated to the left, follows the direction of the “ spina vestibularis”-item of information introduced by Hiss- today holding an ambiguous meaning. It might represent mesenchyme which has migrated from the dorsal mesocardium following the direction of the right pulmonary edge of the septum primum. a Mesenchymal axis, b mesenchymal axis, c dissociation, d septum secundum, e ostium of the coronary sinus, f sinus septi, g septal cusp of the tricuspid valve

Fig. 2.29
Detail of the previous image, in which the two mesenchymal axes are well visible at the base of the interatrial septum. The left axis followed the base of the septum primum, while the right one the septum secundum base. Both are inserting on the tendinous center. These two neighbouring structures formed as a condensation of extracardiac mesenchyme, which are rather hard to identify, explains the extremely frequent confusion between the spina vestibularis and the tendon of Todaro. a Septum secundum, b left atrium, c mesenchymal axis, d ostium of the coronary sinus, e septal cusp of the tricuspid valve
In its anterior-superior part it’s a proper septum and it’s formed by an elongation of the superior endocardial cushion. In the following pictures we tried to underline the structural variability of this septum.
Septum secundum divides the blood flow within the sinus venosus in a main course towards the left atrium and a secondary course, which flows towards the future tricuspid ostium. Due to this fact this septum is also called crista dividens [3].
The superior edge of septum secundum is a veritable crista interveniens. During fetal development, at its level, there can sometimes be found a small prominence named the intercaval tubercle of Lower (Figs. 2.30 and 2.31). This structure guides the blood of the superior vena cava towards the tricuspid valve.
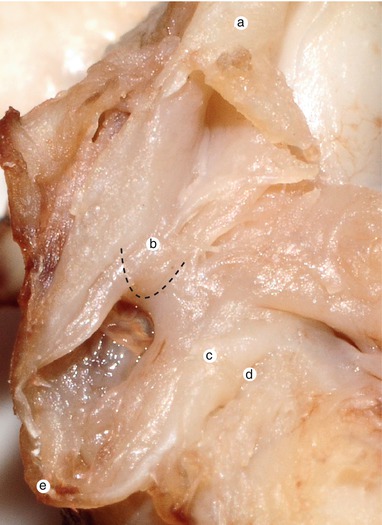
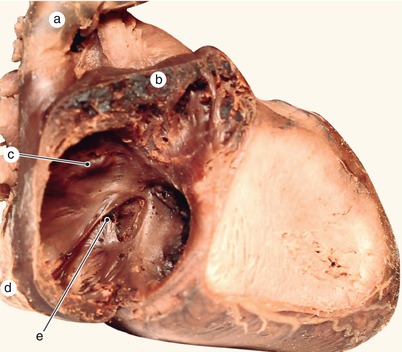

Fig. 2.30
Two and a half months old heart. The right side of the interatrial septum. The intervenous Lower tubercle protrudes on the superior edge of the septum secundum. a Superior vena cava, b the intervenous tubercle on the septum secundum edge, c sinus septi, d ostium of the coronary sinus, e inferior vena cava

Fig. 2.31
A 7 months old heart with Norwood syndrome – left heart hypoplasia. Interatrial communication is prematurely interrupted. Fossa ovalis is bordered superiorly by the intervenous tubercle. a Superior vena cava, b right auricle, c intervenous tubercle – lower on the margin of the septum secundum. Fossa ovalis is closed, d inferior vena cava, e coronary sinus ostium
During evolution:
reptiles have a heart with three chambers and a partially separated ventricle. There is no septum secundum.
birds have a heart with four chambers. There is no septum secundum [4].
Septum secundum occurs in mammals as an evolutional gain in the development of a four-chambered heart.
Septum secundum will become the most massive atrial septum and will form most of the interatrial septum, in the segment which lies superiorly and anteriorly to the fossa ovalis. Septum secundum (a muscular structure), on its’ left side, is covered by the superior limb of septum primum (a membranous structure). Both structures will suffer a variable coalescence process that will lead to the closing of the interatrial conduit.
The occurrence of septum secundum is accompanied by a folding of the atrial wall on the opposite side, therefore septum secundum in the posterior-inferior part looks more like a fold of the atrial wall, to the right of septum primum. The free margin of this fold undergoes a coalescence and a fibromuscular transformation process. So, this margin has a special individual aspect.
Septum secundum is formed only after the left valve of sinus venosus fuses with septum primum. Due to the fact that posteriorly, septum secundum is formed like a fold in the atrial wall, it is considered that this fold rises the left valve while fusing with septum primum. Some authors consider that the left valve fuses with septum secundum (Fig. 2.32).
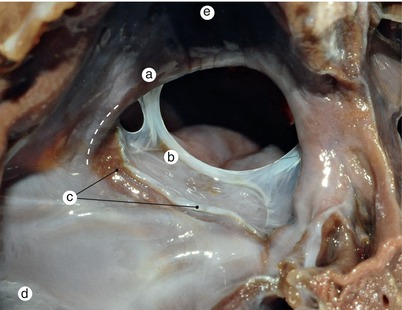

Fig. 2.32
Detailed view of the left valve from the heart in figure 2.20. The fold represented by the posterior limb of the septum secundum bulges in the left valve which has already merged with the septum secundum (dotted line). a Septum secundum, b septum primum, c left valve of the sinus venosus, d inferior vena cava, e superior vena cava
Septum secundum evolves inferiorly. It has the shape of a reversed “U” also facing posteriorly. The free edge of this septum covers on the right side a complex structure, which is the result of fusion of septum primum, vestibular spine and left valve of the sinus venosus.
For conformity:
the edges of fossa ovalis are anterior-superior delimited by septum secundum and posteriorly my a atrial wall fold that continues septum secundum (muscular structure). This fold is considered to be part of septum secundum. These margins will become the limb of fossa ovalis.

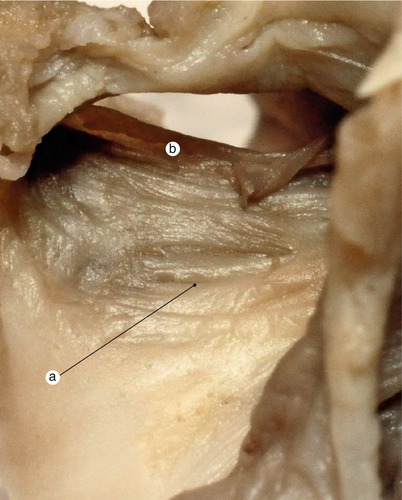
Fig. 2.33
The complex resulted from the fusion of the left valve with the extracardiac mesenchyme and the septum primum is displayed in a normal evolution, in the course of muscularization. a Left valve of the sinus venosus, merged with the septum primum, b septum primum

Fig. 2.34
A 4 months old heart. Detailed view of figure 2.21. Both valves of the sinus venosus in evolution can be detected simultaneously. The sinus septi bulges in the base of the right valve dividing it into the eustachian and thebesian valves. a Superior vena cava, b septum secundum, c septum primum, d left valve of the sinus venosus merged with the septum primum, e right valve, f inferior vena cava, g marginal fold, h coronary sinus ostium, i sinus septi
In fetus, the fossa ovalis isn’t closed. It is just the entrance to an inter-atrial communication. Due to this fact, it is often called foramen ovale, which continues the fossa ovalis and opens in the left atrium. We consider that using the term fossa ovalis in this case is inadequate.
In previous lines we stated that left limb of septum primum faces towards left and there is a space between it and septum secundum.
This statement becomes essential in order to understand that through the vicinity of the fossa ovalis we can enter into a different conduit that is located within interatrial septum. It stretches between septum primum (membranous) and septum secundum (muscular). This conduit exits through an opening delimited by falx septi, atrial wall and septum secundum. This conduit is practically and oblique opening (not a fossa ovalis), which stretches between septum primum and septum secundum. Therefore, due to a higher pressure in the right atrium the falx sepi occurs like an enlargement of the atrial septum within left atrium (Fig. 2.34).
The oblique configuration of this conduit solves the blood dynamic problem we states before: the increased pressure in the left atrium (generated by the functioning pulmonary circulation) compresses falx septi upon septum secundum and the communication between both atria closes. Depending on the fusion grade of structures, a communication conduit between both atria may remain or not (Figs. 2.35 and 2.17).
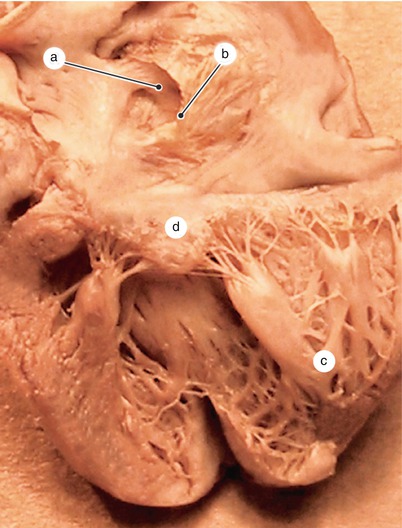

Fig. 2.35
A 4 months and a half old heart. The valve of the fossa ovalis – falx septi seen from the left atrium. a Septum secundum, b falx septi, c posterior papillary group, d anterior mitral cusp
The concave margin of septum secundum will become the limb of fossa ovalis (in the right atrium) and flax septi in the left atrium (see further – Internal aspect of atria).
Final development of atria is made by a strengthening of the posterior-inferior aspect of fossa ovalis and dorsal sinusal space.
This strengthening takes place along with the development of the extraembrionic mesenchyme, fusion between left valve and septum primum, coalescence of septal-oval recess and folding of right atrial wall.
2.2 Interatrial Septal Defects
Lack of dorsal mesoderm development may lead to the persistence of foramen primum, with an endocardial cushion fusion defect and a defect of the anterior mitral cusp. The interventricular septum still develops and this malformation is classically known as foramen primum defect. Therefore this type of defect occurs at the base of the interatrial wall and is accompanied by a partial fusion of endocardial cushions and defects of the anterior mitral cusp.
If septum intermedius does not forms due to a complete lack of endocardial cushion fusion, then an unique atrium with a small partial septum primum communicates with an unique ventricle partially septated. Therefore we can state that there is a persistence of the atrio-ventricular canal. This canal has its’ own valves (five in number) (Figs. 2.36, 2.116 and 2.117).
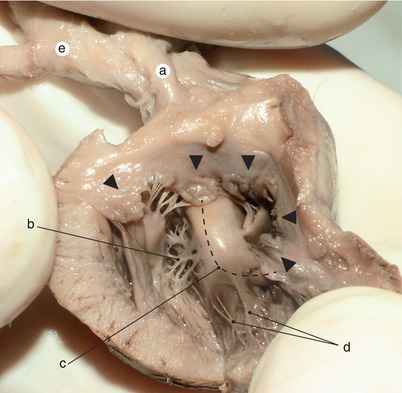

Fig. 2.36
A 6 months old heart. In a left isomerism syndrome we can identify an incompletely divided ventricle and an atrioventricular canal which is bordered by five cusps (indicated by the arrowheads). The detailed view of the image is found in the laterality disorders section. The dotted line indicates the muscular interventricular septum with the superior and the inferior limb. a Atresic pulmonary artery, b left branch of Hiss bundle, c muscular interventricular septum with superior and inferior limb, d right branch of Hiss bundle, e aorta with its origin in the right ventricle
It is often used “fossa ovalis persistence type defect”. In our opinion this definition refers to a mistake we are going to talk about. The definition is based on a variable and persistent communication between left and right atria, communication located between septum secundum and falx septi. We consider that the definition should be: “interatrial persistence conduit defect associated with a lack of fusion with falx septi” (Fig. 2.37).
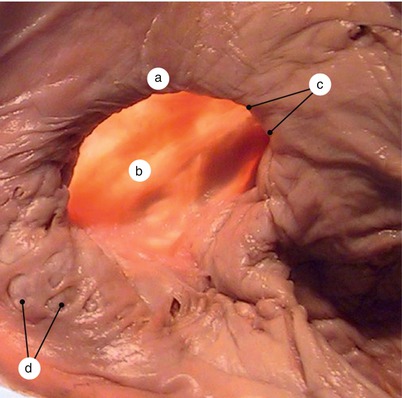

Fig. 2.37
Adult heart. View from the right atrium. Transillumination. Patent foramen ovale through the lack of fusion between falx septi and the septum secundum. a Patent interatrial communication, b the floor of the fossa ovalis is formed by the septum primum, c septum secundum, d reminiscences of the left valve
“Foramen secundum defect type” is based on the presence of at least one septal defect within fossa ovalis area or posterior to it. There is falx septi that can be fused or not with septum secundum (Figs. 2.19 and 2.20). There are parts of the fossa ovalis that can be used for differential diagnosis.
This lack of fusion might be due to a larger foramen secundum that couldn’t be closed by a posterior-inferior development of septum primum or due to a lack of development of septum primum (most probably by a lack of fusion of left valve of sinus venosus) (Fig. 2.38). There can also be apoptotic regions within septum primum that can lead to a foramen secundum outside fusion area between septum primum and left valve of sinus venosus. These defects can be called “fossa ovalis perforation defects”.
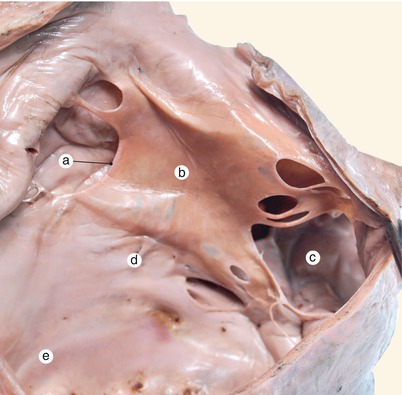

Fig. 2.38
Adult heart. View of the interatrial septum from whithin the left atrium. Falx septi is not merged with the septum secundum, so the interatrial conduit is patent. The orifice secundum has not been compensated and is still patent. Muscularised and rectangular shaped septum primum. The bottom of the inferior limb of the septum primum is correctly merged with the septum intermedium, so there is no ostium primum. a falx septi, b septum primum, c uncompensated ostium secundum, d former septum intermedium, e left atrium
Septum secundum is incompletely closed by falx septi. It happens due to a lack of development of septum primum or an excessive resorption of septum primum anterior to foramen secundum or a septum secundum development defect [5] (from Chap. 1). This type of defect is called “fossa ovalis floor development defect”. This kind of defect has the shape of a fossa ovalis without its floor due to a lack of development. Calling this defect “persistence of oval foramen defect” was just a small step forward. A real oval foramen never existed during development therefore the “persistence of oval foramen” was misattributed to a patent intraseptal conduit.
Sinusal interatrial septal defect is located in the vicinity of sinus venarum.
These defects might be superior caval or inferior caval due to vicinity of caval orifices.
Superior caval sinusal defect is due to a lack of strengthening of interatrial septum in the posterior-superior part of fossa ovalis. This is a secondary orifice that is not compensated by fusion of the septum primum with posterior-superior limb of septum secundum. In this case, the superior right pulmonary vein opens in the right atrium.
Inferior caval sinusal defect represents a lack of strengthening of the posterior-inferior part of fossa ovalis. It is a secondary orifice due to a lack of fusion between septum primum and left valve of sinus venosus (Fig. 2.39).
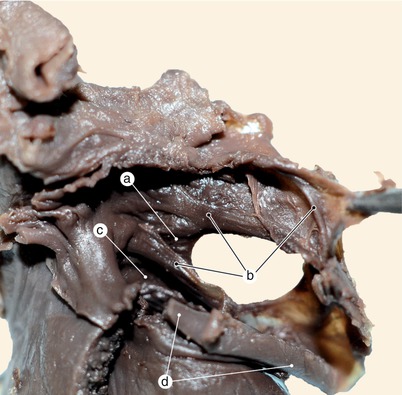

Fig. 2.39
A 4 months old heart. View of the interatrial septum from whithin the left atrium. Interatrial septal sinusal defect that involves the inferior vena cava. The coronary sinus venosus is merged with the inferior vena cava. The posteroinferior side of the interatrial septum has not been yet consolidated. We can distinguish the main elements of the septum primum. a Septum secundum, b septum primum, c mitral orifice, d coronary sinus opened in the inferior vena cava
If we consider the coronary sinus as an extension of the right atrium we can describe another defect – a pathologic interatrial communication due to defects of the coronary sinus roof and the coronary sinus opens in the left atrium.
A premature closing of interatrial communication, before birth, is followed by right heart hypertrophy and left heart hypoplasia. Figures 2.40, 2.41, 2.42 and 2.43 represents Norwood syndrome aspects (left heart hypoplasia).
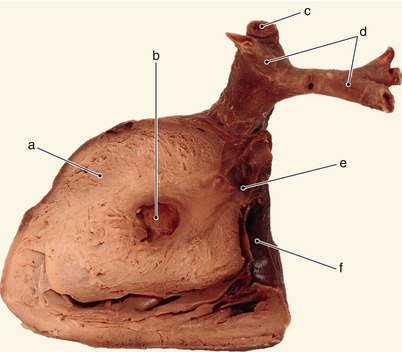
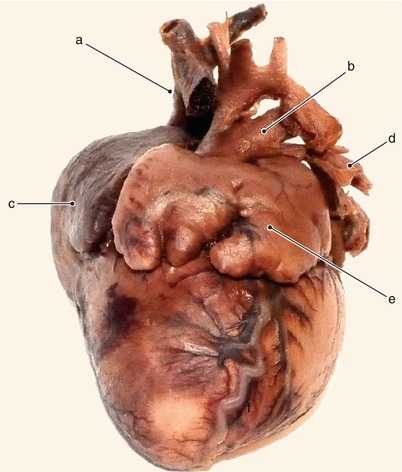
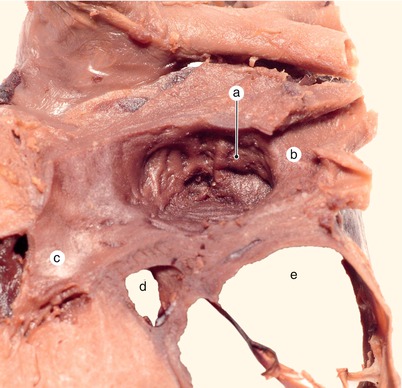
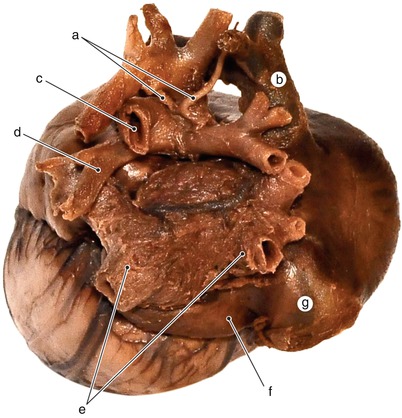

Fig. 2.40
A 8 months old heart. Norwood syndrome (left heart hypoplasia). View from the left of a sagittal section through the left ventricle. a Hypertrophic wall of the left ventricle, b hypoplastic left ventricle cavity, c arterial canal, d pulmonary arteries, e atresic aortic valves, f tricuspid orifice

Fig. 2.41
The same heart. Anterior view. a Superior vena cava, b arterial canal, c right auricle, d left pulmonary artery, e giant left auricle

Fig. 2.42
The same heart. Detailed view from the left of the interatrial septum. The fossa ovalis is prematurely obturated. The left atrium is hypoplastic and the mitral valve is atresic. a Hypoplastic left atrium with no interatrial communication, b pulmonary veins, c atresic mitral cusp, d coronary sinus, e right atrium

Fig. 2.43
The same heart. Posterior view of the base of the heart. Notice the incompletely developed coronary sinus, very suggestive for the expression: the sinus is an exteriorized right atrium. a Reminiscences of bronchial arteries, b superior vena cava, c arterial canal, d left pulmonary artery, e left atrium with pulmonary veins, f left horn of the sinus venosus, g inferior vena cava
2.3 Evolution of the Right Atrium and of the Sinus Venosus
2.3.1 Incorporation of the Right Horn of Sinus Venosus in the Atrial Wall
The main veins of the embryo (umbilical vein, vitelline vein and common cardinal vein) fuse in order to build a venous canal (Cuvier) called sinusal horn (on each side).
Both sinusal horns (right and left) fuse in order to form the primitive sinus venosus (Fig. 2.43).
These horns fuse inferiorly where a crest is formed and is called sinus septi (sinusal septum) (Fig. 2.56).
In the superior part, both horns fuse with the primitive atrium building the sinoatrial part. This part is delimited by the two valves of sinus venosus, that occur in the atrium as two endocardial folds. They are similar with the one from which the septum primum develops.
In the primitive cardiac tube, the sinusal valves are located at the sino-atrial border and are lateral structures. Evaluation of the congenital defects should consider the atrial involvement of the sinus and the lateral position of the valves.
Along with the atrial septation, the left umbilical vein involves, therefore the left atrium becomes smaller than the right one. It follows hemodynamic fall of the left atrium, followed again by an atrial asymmetry and the sinus venosus opens in the right atrium.
The primitive sinus venosus is involved in certain “movements” during development:
Get Clinical Tree app for offline access

A.
The sino-atrial junction suffers a translation movement and changes its position. From an almost transverse position on the longitudinal axis of the cardiac tube it reaches a final position in a frontal plane. In this new position the anterior commissural area is oblique, in contact with the inferior endocardial cushion. The posterior commissure is oblique and faces towards superior and to the right.
Septum spurium is an endocardial fold raised by the fusion of the two valves at the superior commissure. The superior commissure is in contact with the superior endocardial cushion through this structure.
In this position:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


