Figure 4.1
Material removed at pulmonary thromboendarterectomy, courtesy of Dr J Haney, Duke University Medical Center
Reperfusion is followed by a second period of circulatory arrest to allow completion of thromboendarterectomy in the contralateral lung [7]. Additional cardiac procedures can be performed after arteriotomy closure, during rewarming, if necessary (e.g. coronary bypass grafting, foramen ovale closure, mitral valve repair). The use of selective antegrade cerebral perfusion for pulmonary endarterectomy appears to be technically feasible in preliminary trials and is a potential alternative to complete circulatory arrest [8].
The Jamieson intra-operative classification of CTEPH defines patients according to the surgical specimens obtained. There are 4 so called “types”. In type 1 fresh thrombus is present in the main-lobar pulmonary arteries; in type 2 there is intimal thickening and fibrosis proximal to the segmental arteries; in type 3 there is disease within distal segmental arteries only; in type 4 there is distal arteriolar vasculopathy without visible thromboembolic disease (i.e. misdiagnosed IPAH) [9].
There have been no randomised controlled trials to support the insertion of inferior vena cava filters at the time of PTE and the level of evidence for recommending their use is low [2].
Outcomes of PTE
PTE can be performed with low perioperative mortality, with significant improvements in functional capability, hemodynamics and survival. In-hospital mortality is now <5 % in experienced centres [2]. In the majority of cases post-PTE, there is an immediate and sustained fall in pulmonary artery pressure and pulmonary vascular resistance, with a parallel increase in pulmonary blood flow and cardiac output. There is rapid normalisation of right ventricular geometry and tricuspid valve function, such that tricuspid annulopasty is not routinely indicated.
The most significant complications of the procedure are reperfusion pulmonary oedema and persistent pulmonary arterial hypertension. Reperfusion oedema most often develops within 72 h of surgery, and corresponds to anatomic locations distal to where PTE is performed [10]. Mechanical ventilation, inhaled nitric oxide (NO) and intravenous iloprost are advocated to improve the oedema. Up to one third of patients undergoing PTE require >2 days ventilatory support because of reperfusion injury, and this complication is responsible for approximately half the mortality associated with the procedure [11].
Persistent pulmonary hypertension, suggestive of inadequate endarterectomy or underlying small vessel/microvascular disease, is a key determinant of short and long term outcomes. Patients with an elevated post-operative PVR have difficulties weaning from cardiopulmonary bypass, early postoperative hemodynamic instability and early postoperative death, particularly in the context of right ventricular dysfunction [2]. Mortality amongst patients with a postoperative PVR exceeding 500 dyn/s/cm−5 (6.25 WU) is around 30 times greater than for those with a postoperative PVR of less than 500 dyn/s/cm−5. Although postoperative PVR is the greater predictor of mortality, preoperative PVR in excess of 1,000 dyn/s/cm−5 (12.5 WU) has also been associated with poor outcomes [12].
Patients with distal thromboembolic disease (intraoperative classification type 3–4) have higher perioperative mortality, require longer inotropic support, and have longer hospital stays than patients with type 1 or 2 thromboembolic disease [13].
Overall mortality for PTE is reducing with increasing experience of the procedure and is currently less than 5 % [12], with long-term survival exceeding medical therapy or transplantation and persistent improvements in functional status. Four years after PTE around three quarters of patients are in NYHA class I [14].
Surgical Alternatives to PTE
Small scale observational studies in specialist centres have highlighted a potential role for percutaneous pulmonary balloon angioplasty in the management of patients with CTEPH deemed unsuitable for PTE [15, 16]. The technique, which was initially dismissed in the 1980s, has been demonstrated to reduce mean pulmonary artery pressure and effect improved functional capacity. Reperfusion injury is a recognised complication of the procedure and there have been incidences of wiring perforation of the pulmonary vasculature. Systemic and cerebral embolisation are additional hazards. Transplantation remains the definitive surgical alternative for patients with CTEPH not suitable for PTE.
In the acute setting of submassive/massive pulmonary embolism, there is a role for emergency surgical thrombectomy, in preference to thromboendarterectomy. When surgical intervention is contraindicated, percutaneous interventions for removing pulmonary emboli and decreasing thrombus burden include aspiration thrombectomy, thrombus fragmentation, and rheolytic thrombectomy [17].
Atrial Septostomy
Atrial septostomy was originally conceived as a treatment for transposition of the great arteries in neonates [18, 19]. A role for septostomy in the management of pulmonary hypertension was considered when patients with Eisenmenger’s syndrome and idiopathic pulmonary arterial hypertension (IPAH) with a patent foramen ovale were noted to have a survival advantage over those without a patent foramen ovale [20]. The first reported use of atrial septostomy in the palliative treatment of refractory primary pulmonary hypertension was in 1983 [21].
Atrial septostomy involves the formation of an intra-atrial right-to-left shunt, diverting blood flow to bypass the pulmonary vasculature, decompressing the right heart, increasing left ventricular preload and augmenting systemic blood flow (particularly during exercise). The resulting increase in cardiac output enhances tissue perfusion, albeit with a reduced systemic arterial oxygen saturation.
Severe IPAH is the most common indication for atrial septostomy. The procedure has also been used for patients with pulmonary arterial hypertension associated with surgically corrected congenital heart disease, peripheral CTEPH and connective tissue disease.
Patient Selection
Atrial septostomy is recommended only for patients with severe pulmonary arterial hypertension and intractable right heart failure resistant to maximal medical therapy (including inotropic support) [22]. Evidence suggests a benefit in patients in WHO functional class IV with refractory right heart failure or severe syncopal symptoms.
For optimum benefit, the procedure should be performed before there is advanced end-organ dysfunction and haemodynamic compromise. Contraindications to the procedure are the requirement for cardio-respiratory support, mean right atrial pressure >20 mmHg, pulmonary vascular resistance index >55 WU/m2 (where PVRI is defined as the pressure drop across the pulmonary circulation divided by cardiac index), resting oxygen saturation <90 % on room air, and left ventricular end diastolic pressure >18 mmHg [23]. Patient selection therefore requires experience and judgment. If clinically indicated, patients may undergo serial septostomy procedures.
Surgical Technique
Atrial septostomy is performed by surgical incision (the blade technique), graded balloon dilation or a combination of the two. The balloon dilatation technique is preferred as it offers comparable improvements in symptoms and haemodynamics but a lesser procedural risk than the blade technique.
In the balloon dilatation approach, a Swan-Ganz catheter is placed via the right internal jugular vein for haemodynamic monitoring (right atrial pressure, mean pulmonary artery pressure and cardiac index using the thermodilution method). A sheath is passed into the left atrium by needle puncture. Thereafter, a balloon catheter is passed across the septum through the sheath on a guide wire and the sheath withdrawn to the right atrium. The balloon is inflated at low pressures under fluoroscopic guidance. The procedure is repeated with increasing balloon sizes until a septal defect is created that results in a 10 % fall in arterial oxygen saturation [24]. Oxygen delivery is optimised by the transfusion of packed red blood cells or darbepoetin administered pre and post-procedure (Fig. 4.2).
In the blade-balloon approach, balloon dilatation is preceded by the use of a surgical blade, withdrawn across the intra-atrial septum. A total of 3–6 incisions are made at orthagonal angles to achieve a 5–10 % fall in arterial oxygen saturation [26].
The use of intra-cardiac echocardiography to guide the location and extent of septostomy formation has been reported [27]. Recently, a novel septostomy device has been trialled, comprising an atrial septal defect closure device, customised with four 5 mm diameter holes in the central region, but this is not yet in routine use [28].
Outcomes of Atrial Septostomy
There is concern regarding high procedural mortality for septostomy, which is estimated at 16 % but has been reported from 5 to 50 % [29]. Mortality is higher for the blade balloon technique, which carries a greater risk of septal laceration and fatal hypoxaemia. Septostomy is largely restricted to critically ill patients with severe pulmonary hypertension and right ventricular impairment, therefore patient selection contributes to the high mortality. A mean right atrial pressure >20 mmHg, PVR index >55 WU/m2, and an estimated 1-year survival less than 40 % are significant predictors of procedure-related death [29]. Septostomy is primarily a palliative or bridging procedure and reported success rates for bridging patients to transplantation range from 30 to 40 % [30]. Improved cardiac output appears to be the principal hemodynamic benefit. There is an associated symptomatic and functional improvement measured by NYHA class and 6 min walk test [31, 32]. Worldwide experience has demonstrated a median survival of 19.5 months (range, 2–96 months), and late deaths primarily result from progression of underlying pulmonary vascular disease [29].
Surgical Alternatives to Atrial Septostomy
An innovative alternative to septostomy is the Potts shunt procedure, in which needle perforation of the descending aorta is performed at the site of apposition to the left pulmonary artery to create a tract for deployment of a stent between these vessels [33, 34]. The stent acts as a shunt between the pulmonary and systemic circulation, avoiding an intra-cardiac shunt. At present this procedure is limited to the research setting.
Transplantation
The first heart-lung transplant was performed in 1981, for a female patient with IPAH. With the evolution of single and bilateral lung transplant procedures, transplantation is now considered the final definitive treatment for carefully selected patients with advanced pulmonary hypertension. The most common indication is IPAH; less common indications are scleroderma, histiocytosis, and sarcoidosis. Improved disease-specific medical management has reduced the number of patients referred for transplantation; however, around 25 % of patients with IPAH fail to respond to medical therapy.
There is a lack of consensus on the optimal transplantation procedure for patients with pulmonary hypertension. Single lung, bilateral lung and combined heart-lung transplantation have all been performed historically. Single-lung transplantation has been widely discontinued for IPAH due to poor outcomes and International Society for Heart and Lung Transplantation Registry data indicates the majority of pulmonary hypertension patients worldwide receive bilateral lungs. At present, around 5 % of bilateral lung transplants, 3 % of all lung transplants and 28 % of all heart-lung transplants performed worldwide in adults are for IPAH [35].
Patient Selection
Transplantation should be considered and/or discussed with all patients at the time of diagnosis of pulmonary arterial hypertension. Early referral minimises the transplantation of patients with established significant comorbidity. The timing of referral is a recognised challenge given the poor prognosis of patients with disease refractory to medical management and the limited availability of organs.
The International Society for Heart and Lung Transplantation (ISHLT) has published guidelines for the referral and listing of potential transplant candidates. Referral is recommended for patients in NYHA functional class III or IV (irrespective of on-going therapy), or those with rapidly progressive disease. Listing is recommend for patients with persistent functional class III or IV on maximal medical therapy, failure to respond to intravenous epoprostenol (or equivalent), 6 min walk distance <350 m or declining, cardiac index <2 L/min/m2 and right atrial pressure >15 mmHg.
The majority of patients are listed for bilateral lung transplant. Heart-lung transplantation is reserved for patients with intractable right heart failure, especially those who are dependent on inotropic support. Patients with pulmonary hypertension secondary to congenital heart disease (particularly those with Eisenmenger’s) are also more likely to be considered for the combined procedure, although isolated lung transplant may be performed concurrently with cardiac repair. Rarely, the combined procedure is offered to patients with pulmonary hypertension and coexistent advanced left heart disease [36].
Surgical Technique
Anaesthesia in the intended transplant recipient is generally postponed until the donor lungs have been inspected and approved by the retrieval team. The recipient is intubated with a double lumen tube to allow single-lung ventilation. In severe pulmonary hypertension, single-lung ventilation is not attempted. Trans-oesophageal echocardiography is used to monitor right ventricular function and cardiac filling.
Bilateral lung transplantation is typically performed via a transverse thoracosternotomy (clamshell incision). Median sternotomy and bilateral anterolateral thoracotomies with sternal sparing are also used. Bilateral lungs are implanted separately and sequentially. The first lung to be transplanted is generally the one with the least perfusion on V/Q scanning [37].
Cardiopulmonary bypass is often commenced electively in patients with pulmonary arterial hypertension or when concomitant coronary artery bypass graft or cardiac repair is planned. If cardiopulmonary bypass is required for hemodynamic or ventilatory support, the heart remains warm and beating. If cardiac repair is necessary, the heart is arrested and cooled.
Perioperative Considerations
Lung transplantation is associated with an immediate improvement in pulmonary artery pressure with rapid normalisation of right ventricular size and septal geometry [38]. Despite the reduction in right ventricular afterload, right ventricular systolic function and left diastolic function do not improve immediately and haemodynamic instability is common in the early postoperative period. As such, patients with pulmonary arterial hypertension often require temporary inotropic, vasopressor, and inhaled nitric oxide support. Ventricular assist devices are increasingly used to support the right ventricle as it recovers.
In patients without pulmonary hypertension, ventilatory weaning usually occurs over the first few hours to days. In patients with pulmonary hypertension, haemodynamics and oxygenation are more labile, especially following single lung transplantation. A more cautious weaning approach is adopted with the continuation of neuromuscular paralysis, sedation, and ventilatory support for 24–48 h postoperatively. Thereafter, paralysis and sedation are gradually reversed and weaning follows.
Within the first 72 h post-transplant, primary graft dysfunction is a significant concern in patients with pulmonary arterial hypertension, and is associated with increased risk of death [39]. Risk factors for primary graft dysfunction include intraoperative hemorrhage or cardiovascular complications, ischemia-reperfusion lung injury, and the use of cardiopulmonary bypass in the context of severe right ventricular dysfunction. Ischaemia-reperfusion injury is more prevalent following single lung transplantation, when there is a preponderance of blood flow to the allograft lung in response to high pulmonary vascular resistance in the native lung. Any compromise to the allograft (e.g. infection, rejection) can therefore result in severe ventilation-perfusion mismatch [40]. Bilateral lung transplantation results in a lesser degree of ventilation-perfusion mismatch and these patients are easier to care for in the perioperative period.
Outcomes of Transplantation
Survival after lung and heart-lung transplantation for IPAH has historically been lower than for other major diagnostic categories of lung transplant recipients, although higher than for idiopathic pulmonary fibrosis. Recent data suggests that although patients with IPAH undergoing transplantation have an increased 3 month mortality, their long term outcomes are comparable with patients with other diagnoses [35, 41].
The ISHLT registry reports 1-, 3-, 5-, and 10-year survival of 66 %, 57 %, 47 %, and 27 %, respectively, in pulmonary arterial hypertension patients undergoing lung transplantation [42]. These compare with baseline survival rates of 79, 64, 53, and 30 % for all lung transplant procedures [35] (Fig. 4.3).
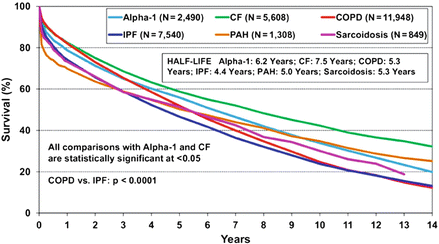

Figure 4.3
Kaplan-Meier survival by diagnosis for adult lung transplants performed between January 1990 and June 2010 [35]. (Alpha-1 α1-antitrypsin deficiency emphysema, CF cystic fibrosis, COPD chronic obstructive pulmonary disease, IPF idiopathic pulmonary fibrosis, PAH pulmonary arterial hypertension)
ISHLT registry data demonstrates improved survival amongst all patients receiving bilateral rather than single lung transplant [35] and this is especially the case for IPAH. To date, there have been no direct comparisons of lung transplant versus heart-lung transplant in this population. In patients with Eisenmenger’s syndrome secondary to ventricular septal defect, there appears to be a survival benefit with combined heart-lung transplant over bilateral lung transplant with simultaneous closure of the defect.
The incidence of postoperative obliterative bronchiolitis appears to be higher in patients with IPAH and the process onsets sooner than for other conditions necessitating transplantation [43]. Recurrence of IPAH after transplantation has not been reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree