with a corresponding compression of the contralateral vessel wall. An additional artifact, transducer ring-down, appears in virtually all medical ultrasound devices. This artifact arises from acoustic oscillations in the piezoelectric transducer material and results in high-amplitude signals that obscure near-field imaging. In mechanical systems, this artifact may be merged with the imaging sheath artifact. In electronic array catheters, this artifact may be removed largely by mask subtraction. All intravascular imaging systems are vulnerable to the geometric distortion produced by oblique imaging. Thus, when the ultrasound beam interrogates a plane not orthogonal to the vessel walls, an artery with a circular lumen appears elliptical in shape. Some transducer designs position the guide wire external to the transducer, thereby introducing an obligatory wire artifact. In general, higher frequency transducers have a lower penetration depth; in practice, this is not usually an issue for coronary imaging, but may become evident if peripheral arterial imaging is attempted. Alternative catheters with reduced frequency are used for large-vessel peripheral imaging and for intracardiac examination.
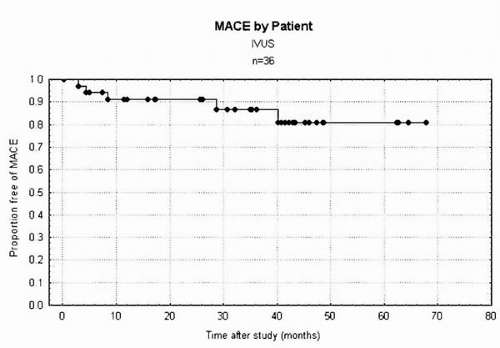
70%) in patients whose symptomatic status is difficult to evaluate. For these ambiguous lesions, ultrasound provides tomographic measurements, enabling the quantification of the stenosis independent of the radiographic projection (36,21). Bifurcation lesions are particularly difficult to assess by angiography because overlapping side branches often obscure the lesion (37). Intravascular lesion cross-sectional areas have shown a statistically significant, although weak, correlation with noninvasive stress imaging studies and other measures of stenosis severity (38, 39, 40, 41, 42). In general, lumen areas less than 3 to 4 mm2 correlate with a positive stress study. Our own longitudinal study of 36 patients in whom IVUS was performed to assess lesion significance and for whom treatment was deferred showed a 14.3% major adverse cardiac event rate at a mean follow-up of 18.8 months (Fig. 5A.1). Generally, however, functional measures such as fractional flow reserve may be better suited for assessing the prognostic significance of intermittent lesions.
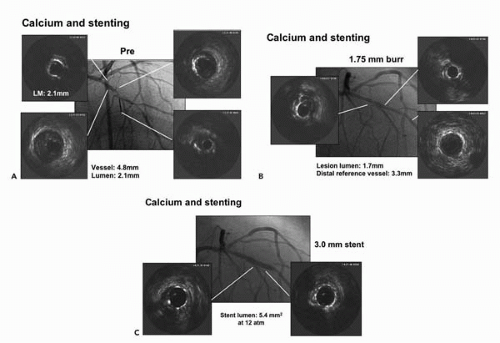
minimal stent area. Areas over 8 to 10 mm2 are generally associated with target lesion revascularization rates of 10% or less (53, 54, 55, 56). Although inadequate minimal stent area is the predominant factor associated with in-stent restenosis lesions, nearly 5% of cases are found to have significant mechanical implantation abnormalities (57). Many experts recommend that all cases of in-stent restenosis be interrogated by IVUS to define the mechanism and guide therapy.
TABLE 5A.1. IVUS- VERSUS ANGIOGRAPHICALLY-GUIDED PCI TRIALS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree