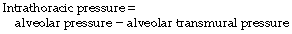Chapter 7 The pulmonary circulation
 Pulmonary blood flow approximates to cardiac output, and can increase several-fold with little change in pulmonary arterial pressure.
Pulmonary blood flow approximates to cardiac output, and can increase several-fold with little change in pulmonary arterial pressure. Passive distension and recruitment of closed pulmonary capillaries, particularly in the upper zones of the lung, allow pulmonary vascular resistance to fall as blood flow increases.
Passive distension and recruitment of closed pulmonary capillaries, particularly in the upper zones of the lung, allow pulmonary vascular resistance to fall as blood flow increases. Active control of pulmonary vascular resistance has only a minor role in controlling pulmonary vascular resistance and involves intrinsic responses in vascular smooth muscle, modulated by numerous neural and humoral factors.
Active control of pulmonary vascular resistance has only a minor role in controlling pulmonary vascular resistance and involves intrinsic responses in vascular smooth muscle, modulated by numerous neural and humoral factors.Evolution first led to the development of a separate pulmonary circulation in amphibians, though in this case both systemic and pulmonary circulations are supplied by a single ventricle and there is therefore a great deal of mixing of blood between the two. The occurrence of warm-blooded animals led to a 10-fold increase in oxygen requirements, which may only be achieved through having a pulmonary circulation almost completely separate from the systemic circulation.1
Pulmonary Blood Flow
The flow of blood through the pulmonary circulation is approximately equal to the flow through the whole of the systemic circulation. It therefore varies from about 6 l.min−1 under resting conditions, to as much as 25 l.min−1 in severe exercise. It is remarkable that such an increase can normally be achieved with minimal increase in pressure. Pulmonary vascular pressures and vascular resistance are much less than those of the systemic circulation. Consequently the pulmonary circulation has only limited ability to control the regional distribution of blood flow within the lungs and is markedly affected by gravity, which results in overperfusion of the dependent parts of the lung fields. Maldistribution of the pulmonary blood flow has important consequences for gaseous exchange, and these are considered in Chapter 8.
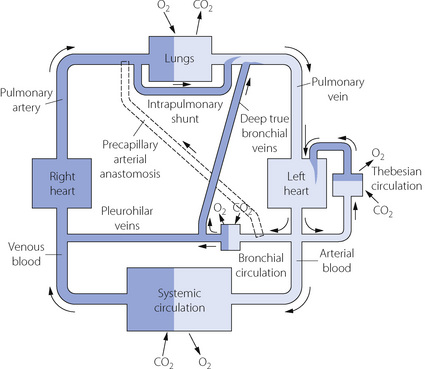
In fact, the relationship between the inflow and outflow of the pulmonary circulation is much more complicated (Figure 7.1). The lungs receive a significant quantity of blood from the bronchial arteries, which usually arise from the arch of the aorta. Blood from the bronchial circulation returns to the heart in two ways. From a plexus around the hilum, blood from the pleurohilar part of the bronchial circulation returns to the superior vena cava via the azygos veins, and this fraction may thus be regarded as normal systemic flow, neither arising from nor returning to the pulmonary circulation. However, another fraction of the bronchial circulation, distributed more peripherally in the lung, passes through postcapillary anastomoses to join the pulmonary veins, constituting an admixture of venous blood with the arterialised blood from the alveolar capillary networks.
The situation may be further complicated by blood flow through precapillary anastomoses from the bronchial arteries to the pulmonary arteries. These communications (so-called ‘sperr arteries’) have muscular walls and are thought to act as sluice gates, opening when increased pulmonary blood flow is required. Their functional significance in normal subjects is unknown, but in diseased lungs flow through these anastomoses may be crucial. For example, in situations involving pulmonary oligaemia (e.g. pulmonary artery stenosis, pulmonary embolism) blood from the bronchial arteries will flow through the anastomoses to supplement pulmonary arterial flow.2 It should be noted that a Blalock–Taussig shunt operation achieves the same purpose for palliation of patients with cynanotic congenital heart disease.
Pulmonary Blood Volume
Factors Influencing Pulmonary Blood Volume
Systemic vascular tone. Because the systemic circulation has much greater vasomotor activity than the pulmonary circulation, an overall increase in vascular tone will tend to squeeze blood from the systemic into the pulmonary circulation. This may result from the release of endogenous catecholamines, administration of vasoconstrictor drugs, or from passive compression of the body in a G-suit. The magnitude of the resulting volume shift will depend on many factors such as position, overall blood volume and activity of the numerous humoral and nervous mechanisms controlling pulmonary vascular tone at the time (see below). Conversely, it seems likely that pulmonary blood volume would be diminished when systemic tone is diminished, as for example during sepsis or with regional anaesthesia when systemic vascular resistance is decreased with no effect on the autonomic supply to the pulmonary vasculature.
Pulmonary Vascular Pressures
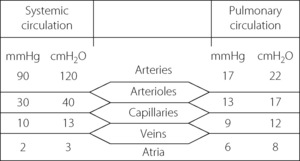
Pulmonary arterial pressure is only about one-sixth of systemic arterial pressure, although the capillary and venous pressures are not greatly different for the two circulations (Figure 7.2). There is thus only a small pressure drop along the pulmonary arterioles and therefore a reduced potential for active regulation of the distribution of the pulmonary blood flow. This also explains why there is little damping of the arterial pressure wave, and the pulmonary capillary blood flow is markedly pulsatile.
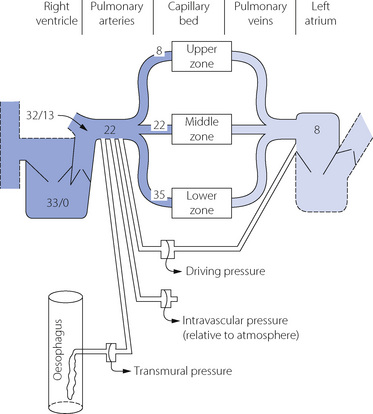
Consideration of pulmonary vascular pressures carries a special difficulty in the selection of the reference pressure. Systemic pressures are customarily measured with reference to ambient atmospheric pressure, but this is not always appropriate when considering the pulmonary arterial pressure, which is relatively small in comparison with the intrathoracic and pulmonary venous pressures. This may be important in two circumstances. First, the extravascular (intrathoracic) pressure may have a major influence on the intravascular pressure and should be taken into account. Secondly, the driving pressure through the pulmonary circulation may be markedly influenced by the pulmonary venous pressure, which must be taken into account when measuring pulmonary vascular resistance. We must therefore distinguish between pressures within the pulmonary circulation expressed in the three different forms listed below. Measurement techniques may be adapted to indicate these pressures directly (Figure 7.3).
Transmural pressure is the difference in pressure between the inside of a vessel and the tissue surrounding the vessel. In the case of the larger pulmonary vessels, the outside pressure is the intrathoracic pressure (commonly measured as the oesophageal pressure, as shown in Figure 7.3). This method should be used to exclude the physical effect of major changes in intrathoracic pressure.
These differences are far from being solely academic. For example, an increase in intrathoracic pressure due to positive pressure ventilation will increase the pulmonary arterial intravascular pressure, but will also similarly increase pulmonary venous intravascular pressure and therefore driving pressure (and therefore flow) remains unchanged. Similarly, if the primary problem is a raised left atrial pressure, blood will ‘back-up’ through the pulmonary circulation and pulmonary arterial intravascular pressure will also be raised but the driving pressure will again not be increased. Therefore for assessing pulmonary blood flow (and so resistance) driving pressure is the correct measurement, but this requires pulmonary venous (left atrial) pressure to be recorded, which is difficult to achieve continuously (page 113). Pulmonary arterial intravascular pressure is usually measured and the value must therefore be interpreted with caution.
Typical normal values for pressures within the pulmonary circulation are shown in Figure 7.3. The effect of gravity on the pulmonary vascular pressure may be seen, and it will be clear why pulmonary oedema is most likely to occur in the lower zones of the lungs where the intravascular pressures and the transmural pressure gradients are highest.
Effect of Intra-Alveolar Pressure
Alveolar transmural pressure is a function of lung volume (Figure 3.8) and, when the lungs are passively inflated, the intrathoracic pressure will normally increase by rather less than half the inflation pressure. The increase will be even less if the lungs are stiff, and thus a low compliance protects the circulation from inflation pressure (page 480). Intravascular pressures are normally increased directly and instantaneously by the effects of changes in intrathoracic pressure, and this explains the initial rise in systemic arterial pressure during a Valsalva manoeuvre (page 478). It also explains the cyclical changes in pulmonary arterial pressure during spontaneous respiration, with pressures greater during expiration than during inspiration. Such changes would not be seen if transmural pressure were measured (Figure 7.3).
Pulmonary Vascular Resistance
There are, however, important caveats and the concept of pulmonary vascular resistance is not a simple parallel to Ohm’s law, appropriate to laminar flow (page 43). First, the tubes through which the blood flows are not rigid but tend to expand as flow is increased, particularly in the pulmonary circulation with its low vasomotor tone. Consequently the resistance tends to fall as flow increases and the plot of pressure against flow rate is neither linear (see Figure 4.2) nor curved with the concavity upwards (see Figure 4.3) but curved with the concavity downwards. The second complication is that blood is a non-Newtonian fluid (due to the presence of the corpuscles) and its viscosity varies with the shear rate, which is a function of its linear velocity.
Vascular resistance in the lung. Although the relationship between flow and pressure in blood vessels is far removed from simple linearity, there is a widespread convention that pulmonary vascular resistance should be expressed in a form of the equation above. This is directly analogous to electrical resistance, as though there were laminar flow of a Newtonian fluid though rigid pipes. It would, of course, be quite impractical in the clinical situation to measure pulmonary driving pressure at different values of cardiac output to determine the true nature of their relationship.
Localisation of the pulmonary vascular resistance. In the systemic circulation the greatest part of resistance is in the arterioles, along which the pressure falls from a mean value of about 12 kPa (90 mmHg) down to about 4 kPa (30 mmHg) (see Figure 7.2). This pressure drop largely obliterates the pulse pressure wave, and the systemic capillary flow is not pulsatile to any great extent. In the pulmonary circulation, the pressure drop along the arterioles is very much smaller than in the systemic circulation and, as an approximation, the pulmonary vascular resistance is equally divided between arteries, capillaries and veins. Pulmonary arteries and arterioles, with muscular vessel walls, are mostly extra-alveolar and involved in active control of pulmonary vascular resistance by mechanisms such as nervous, humoral or gaseous control. In contrast, pulmonary capillaries are intimately associated with the alveolus (see Figure 2.7) so resistance of these vessels is therefore greatly influenced by alveolar pressure and volume. Thus in the pulmonary circulation, vessels without the power of active vasoconstriction play a major role in governing total vascular resistance and the distribution of the pulmonary blood flow.
Passive Changes in Pulmonary Vascular Resistance
Effect of Pulmonary Blood Flow (Cardiac Output)
Recruitment of previously unperfused pulmonary vessels occurs in response to increased pulmonary flow. This is particularly true of the capillary bed, which is devoid of any vasomotor control, so allowing the opening of new passages in the network of capillaries lying in the alveolar septa, and is most likely to occur in the upper part of the lung where capillary pressure is lowest (zone 1, see below). Capillary recruitment was first described in histological studies involving sections cut in lungs rapidly frozen while perfused with blood, which showed that the number of open capillaries increased with rising pulmonary arterial pressure, particularly in the mid-zone of the lung.3 Recruitment of capillaries in the intact lung remains poorly understood. Studies using colloidal gold particles in the circulation demonstrate that there is perfusion in all pulmonary capillaries, including in zone 1, during normal ventilation.4 A similar study, this time with airway pressure increased above pulmonary capillary pressure, showed no flow in almost two-thirds of capillaries in zone 1.5 It therefore seems that with increased alveolar pressure unperfused capillaries are available for recruitment but that under normal circumstances, with low airway pressures, there is flow in all capillaries. However, these studies using colloidal particles cannot discriminate between plasma or blood flow and have led to speculation that some, almost collapsed, capillaries may contain only plasma (‘plasma skimming’) or even blood flow from the bronchial circulation.6
Distension in the entire pulmonary vasculature occurs in response to increased transmural pressure gradient, and is again most likely to occur in capillaries devoid of muscular control. In one study, capillary diameter increased from 5 to 10 μm as the transmural pressure increased from 0.5 to 2.5 kPa (5 to 25 cmH2O).7 As described in the previous section it now seems likely that capillaries never collapse completely and therefore passive distension is clearly the more important adaptation to increased flow.
A striking example of the ability of the pulmonary vasculature to adapt to changing flow occurs after pneumonectomy (page 494), when the remaining lung will normally take the entire resting pulmonary blood flow without a rise in pulmonary arterial pressure. There is, inevitably, a limit to the flow that can be accommodated without an increase in pressure, and this will be less if the pulmonary vascular bed is affected by disease or surgery. The most important pathological cause of increased pulmonary blood flow is left-to-right shunting through a patent ductus arteriosus or through atrial or ventricular septal defects. Under these circumstances the pulmonary blood flow may be several-fold greater than the systemic flow before pulmonary hypertension develops. Despite this, remodelling of the pulmonary vessels commonly results in an increase in vascular resistance, causing an earlier and more severe rise in pulmonary arterial pressure.
Effect of Lung Inflation
Reference has been made above to the effect of alveolar pressure on pulmonary vascular pressures. The effect on pulmonary vascular resistance is complex. Confusion has arisen in the past because of failure to appreciate that pulmonary vascular resistance must be derived from driving pressure and not from pulmonary arterial or transmural pressure (Figure 7.3). This is important because inflation of the lungs normally influences the pressure in the oesophagus, pulmonary artery and left atrium and so can easily conceal the true effect on vascular resistance.
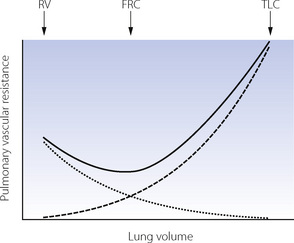
When pulmonary vascular resistance is correctly calculated from the driving pressure, there is reasonable agreement that the pulmonary vascular resistance is minimal at FRC and that changes in lung volume in either direction cause a small increase in resistance, particularly at high lung volumes (Figure 7.4). These observations may be explained by considering pulmonary capillaries as belonging to three distinct groups:8
Alveolar capillaries are sandwiched between two adjacent alveolar walls, usually bulging into one alveolus (see Figure 2.7), and supported from collapse only by the pressure in the capillary and flimsy septal fibrous tissue. Expansion of the alveolus will therefore compress these capillaries and increase their contribution to pulmonary vascular resistance. If the lung consisted entirely of alveolar capillaries then pulmonary vascular resistance would be directly related to lung volume.
Extra-alveolar vessels provide an additional explanation for the increased pulmonary vascular resistance at small lung volumes. Compression of larger pulmonary vessels at low lung volumes may result in reduced flow in dependent parts of the lung (page 123), and this is likely to contribute to the overall change in pulmonary vascular resistance.
Effect of Gravity on Alveolar and Vascular Pressures
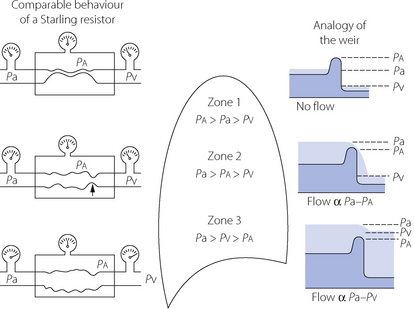
The vascular weir. The interplay of alveolar pressure, flow rate and vascular resistance is best considered by dividing the lung field into three zones.9Figure 7.5 shows behaviour as a Starling resistor and also the analogy of a weir. A Starling, or threshold, resistor can be visualised as a length of compressible tubing within a rigid chamber, such that flow occurs only when the upstream pressure (left gauges in Figure 7.5) exceeds the pressure within the chamber (middle gauges) and a reduction in the downstream pressure (right gauges) cannot initiate flow. In zone 1 of Figure 7.5, the pressure within the arterial end of the collapsible vessels is less than the alveolar pressure, and therefore insufficient to open the vessels that remain collapsed as in a Starling resistor. The upstream water is below the top of the weir and so there can be no flow. The downstream (venous) pressure is irrelevant. Zone 1 corresponds to conditions that may apply in the uppermost parts of the lungs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree