Fig. 22.1
Weekly admissions of patients with poliomyelitis to the Blegdam Hospital in Copenhagen in July to December 1952. Note the very rapid increase in the first part of August. The figure also shows that about half of the patients had some paralysis. (From Lassen [20])
The epidemic resulted in two enormous challenges in applied physiology. The hospital lacked ventilators. The stunningly innovative solution was to use manual positive pressure administered by a roster of 200 medical students who repeatedly squeezed a rubber bag attached to a tracheostomy tube around the clock. The second challenge was understanding the life-threatening abnormalities of pulmonary gas exchange and acid-base status. At the start of the epidemic, the only laboratory test available was the total carbon dioxide concentration of the blood, and the high values were interpreted as a mysterious “alkalosis.” Aspects of the epidemic are discussed elsewhere [3, 21, 22, 29, 31, 34, 35].
The problems posed by the epidemic are interesting in their own right. However, the thesis here is that the situation was symptomatic of the parlous state of clinical physiology in the early 1950s. Great advances in respiratory physiology had been made in the 1940s, partly in response to the demands of World War II, but many had not been translated into the clinical setting. The coverage of respiratory physiology in medical student textbooks around 1950 was generally abysmal.
But in the early part of the decade a renaissance occurred that can be exemplified by the publication in 1955 of The Lung by Julius Comroe and his coauthors [9]. Indeed the decade ushered in a revolution in applied respiratory physiology that lasted for much of the remainder of the century.
22.1 The Poliomyelitis Epidemic
Mechanical ventilation. In the initial stages of the epidemic there was some confusion about the need to ventilate patients with bulbar polio and respiratory insufficiency. Some patients were said to have polioencephalitis or “cerebralia” with a constellation of symptoms and signs, including haziness of consciousness, increased secretions in the airways, long periods of apnea punctuated by occasional inspirations, and obtunding of consciousness from which they could be aroused by verbal stimulation. The relative importance of hypoxia, carbon dioxide retention, fever, and uremia as a cause of this condition was debated [22]. There was an impression that some patients had an overwhelming viral bulbar infection for which little could be done, and this led to therapeutic nihilism [21]. This group had a very high mortality, and, in retrospect, many of these patients should probably have been ventilated much earlier than they were, if indeed they were ventilated at all.
When it was recognized that many of these patients could not survive without mechanical ventilation, the lack of machines became a serious crisis. At the outbreak of the epidemic the Blegdam Hospital had only one Emerson tank respirator and six cuirass respirators. The latter consisted of jackets that fitted around the chest and assisted ventilation by changing the pressure outside the thorax. Although these were useful for patients with mild respiratory impairment, they were utterly inadequate for cases with respiratory paralysis.
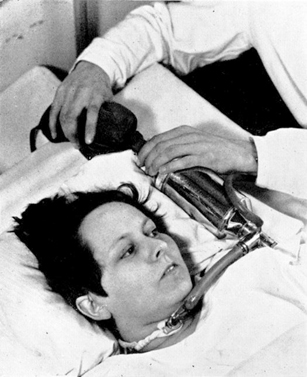
The bold solution was to manually ventilate the patients by squeezing a rubber bag attached to a tracheostomy tube inserted through an incision just below the larynx. The bag was connected to a tank of 50 % oxygen in nitrogen together with a soda lime absorber to remove carbon dioxide (Figs. 22.2 and 22.3). The logistical problem was solved by having a roster of 200 medical students who operated in relays. At the height of the epidemic, 70 patients had to be manually ventilated around the clock. The medical students worked 6–8 h shifts so that three or four shifts were needed in the 24 h. It is daunting to think of the responsibility of these students who were essentially ventilating blind with only the patient’s appearance to guide them, at least in the initial stages. One account refers to a patient rolling her eyes up to signal that she needed more ventilation. Nevertheless the mortality rate is said to have dropped from ~ 90 to ~ 25 % as a result of this heroic intervention.

Fig. 22.2
Apparatus for manual mechanical ventilation. The tank was 50 % O2− 50 % N2. At the bottom is the cuffed endotracheal tube that was inserted through a tracheotomy. (From Lassen [20])
The demands were so great that the supply of medical students dwindled, and a number of lectures were given at the university to encourage more to volunteer [24]. Students from the dental school were also recruited. One report states that 1500 students in all took part in this activity with a total of 165,000 h [17].
The introduction of manual bag ventilation early in the epidemic was due to the anesthesiologist Bjørn Ibsen (born: 1915) (Fig. 22.4). He was a Dane who had spent a period in the Department of Anesthesia at the Massachusetts General Hospital under Henry Knowles Beecher (1904–1976). Earlier in 1952, Ibsen had been involved in the treatment of a child with tetanus who was curarized and ventilated manually through a tracheostomy [35]. There was a dramatic meeting at the Blegdam Hospital on August 25, 1952, when, as stated earlier, 31 patients with bulbar polio had been treated with the tank and cuirass respirators in the preceding 3 week but 27 had died. On that day alone, four patients were autopsied, one of them a 12-year-old boy who died with a “bicarbonate level in the serum far above the normal level.” The attendees included Lassen, Poul Astrup (1915–2000) (Fig. 22.5), chief of the hospital laboratory, Mogens Bjørneboe, a member of the hospital’s medical staff, and Ibsen, who had apparently somewhat reluctantly been invited to attend by Lassen at the urging of Bjørneboe. Ibsen soon recognized that the high blood bicarbonate levels were not an alkalosis of unknown origin but were caused by severe carbon dioxide retention ([3], p. 258). He recommended immediate manual ventilation via a tracheostomy. It is interesting that no successful treatment by positive pressure ventilation given continuously over a long period had been reported by 1950 [12].

Fig. 22.4
Bjørn Ibsen (1915-), Danish anesthesiologist who suggested positive pressure ventilation for the patients with respiratory paralysis. (From Zorab [37])

Fig. 22.5
Poul Astrup (1915–2000) who ushered in the modern period of clinical acid-base physiology. (From Severinghaus and Astrup [28])
Ibsen related how the first patient was a 12-year-old girl who had paralysis of all four extremities, had atelectasis of the left lung, and who was gasping for air and drowning in her own secretions [1, 18]. She was pyrexic, cyanotic, and sweating. A tracheotomy was done under local anesthesia, a cuffed endotracheal tube was placed, and she was eventually ventilated satisfactorily. This event was a turning point in critical care medicine, partly because it was one of the first occasions when an anesthesiologist moved out of the operating room into another environment. Positive pressure ventilation had previously been used for short periods in a polio epidemic in Los Angeles in 1948–1949 [7, 8], but this work had been published in an obscure journal and was not well known.
The ventilation circuit shown in Figs. 22.2 and 22.3 was a semi-closed system similar to that used in many anesthetic machines with the advantage that it required less fresh gas from the tank than an open circuit. The physicians also felt that the buffering effect of the large volume of the circuit made it easier for inexperienced medical students to maintain the appropriate ventilation. The flow meter was set to 5–10 L min. However, the CO2 absorber incorporated in the circuit caused some problems because of potential aspiration of soda lime particles into the lung. In later versions, the absorber was removed from the circuit and there was simply an inspiratory/expiratory valve at the tracheostomy tube so that expired gas was vented directly into the outside air. This required a higher flow rate from the oxygen/nitrogen tank.
It is interesting to look back at a contemporary discussion of the principles of mechanical ventilation in the clinical setting, for example, Rattenborg [27]. There was confusion about the mode of action of positive pressure vs. negative pressure ventilation, and the role of airway resistance on the one hand and lung and chest wall compliance on the other in limiting inflation of the lung. The analysis included a curious statement that negative pressure inflation was unsatisfactory because it did not ensure a constant rate of inspiratory airflow. The reader today is aware of the great contrast between this discussion and the work done in departments of physiology a few years before when the relevant principles of the mechanics of respiration had been clearly enunciated by such groups as Fenn, Otis, and Rahn in the University of Rochester, New York [13], and Mead and Whittenberger and their colleagues at the Harvard School of Public Health [23]. This is an example of the prevailing dissociation in the early 1950s between the advances made in departments of physiology on the one hand and their application to clinical situations on the other.
Pulmonary gas exchange and acid-base status. As indicated earlier, a major difficulty in the successful ventilation of these patients was the almost complete lack of laboratory data about pulmonary gas exchange and acid-base status. The clinical symptoms and signs of respiratory insufficiency were vague or simply caused by intense anxiety. Patients felt suffocated and had difficulty in coping with their secretions because they could not swallow, there was cyanosis if they were not being given oxygen, and a clammy skin and hypertension were sometimes seen, presumably the results of increased blood catecholamine levels. The only routine laboratory investigation available was the total carbon dioxide concentration in venous or arterial blood. The clinical laboratory of the Blegdam Hospital had a pH meter that could be used for blood, but it required a large sample volume and could not be used for frequent or routine measurements. As stated earlier, the very high levels of carbon dioxide concentration were initially attributed to a mysterious alkalosis, and it was Ibsen who first recognized at the time of the epidemic that instead they signaled a severe respiratory acidosis although this had previously been suggested [25].
Astrup related that “this outright misinterpretation of a high CO2 content as alkalosis in patients with respiratory insufficiency produced a very deep impression on me as a laboratory man” (3], p. 258). Astrup was able to persuade the Radiometer A/S in Copenhagen to provide him with a smaller pH meter that could be used for blood [28]. A quick measurement of blood pH at 38 °C soon proved Ibsen right, and this led to the 12-year-old girl being tracheotomized and given manual positive pressure ventilation that immediately caused the “alkalosis” to disappear. Astrup noted that the value of the carbon dioxide concentration of blood as an index of its alkalinity could be traced all the way back to 1877 when it was described by Friedrich Walter (born: 1850) and in its time was a very valuable contribution, but clearly here was a situation where the concept was misleading. In defense of the misconceptions of the clinicians in the early 1950s, it should be added that it was unusual to request laboratory data in patients with abnormalities of ventilation.
A top priority was to measure the PCO2 in the blood, and this was done using the Henderson-Hasselbalch equation. The graphical depiction of this by Van Slyke and Sendroy [33] was well known to Astrup, and their original diagram is reproduced in Fig. 22.6. The vertical line on the extreme left shows the total carbon dioxide content of blood both in milliliters of CO2 per deciliter of blood and in millimolar concentrations, whereas the central and right-hand lines show the plasma pH and PCO2, respectively. A line has subsequently been added joining the normal pH of 7.4 and normal PCO2 of 40 mmHg to show a total CO2 content of about 56 ml CO2 per deciliter of blood.

Fig. 22.6
Nomogram that was used for determining the blood PCO2 from the total CO2 content and plasma pH. (From Van Slyke and Sendroy [33])
The carbon dioxide concentration was determined using the manometric method described by Van Slyke and O’Neill [32]. As mentioned earlier, the normal laboratory blood pH meter at the time required a large sample size. However, when the smaller pH meter became available from Radiometer, more than 700 pH determinations were made in the Blegdam Hospital over the next 4 months [4].
Nevertheless this method for determining the PCO2 of blood was still cumbersome because the Van Slyke manometric method was so time consuming. Astrup later realized that if a sample of either plasma or whole blood was exposed to different CO2 partial pressures, the resulting change in pH was linearly related to the logarithm of the PCO2 within the clinical range [2]. First the pH of the sample of plasma or blood was measured. Then the sample was exposed to gas with high and low PCO2 values (for example, about 80 and 15 mmHg), and the pH for each PCO2 was measured. The actual PCO2 was then obtained by interpolation. This rapid interpolation method was extensively used until the CO2 electrode was eventually introduced several years later.
The early measurements of pH and PCO2 on the patients from the Copenhagen epidemic who were manually ventilated sometimes showed dramatic changes within short periods of time. Table 22.1 shows an example from a 5-year-old boy who was almost moribund on admission and then was tracheotomized and manually ventilated [4]. Note that the pH rose from 6.99 to 7.65 over a 3.5 h period and the PCO2 fell from 150 (in venous blood) to 14 mmHg (in arterial blood)!
In another patient, a woman aged 30 year, the pH and PCO2 were monitored over a series of 13 days (Table 22.2). The fact that a number of the measurements were made on venous rather than arterial blood complicates the interpretation somewhat, but it can be seen that the PCO2 remained fairly stable in the low 30 s until September 10 when, in a venous sample, it had fallen to 17 mmHg! This was the result of a decision made at a conference on September 10 to increase the rate of manual ventilation in all patients from 20 to 30 breaths/min. However, because a number of patients subsequently showed very low PCO2 values, it was then decided to reduce the ventilation frequency to 25 breaths/min. In the patient shown in Table 22.2, the PCO2 then rose to 23 mmHg.
Note that all these PCO2 values were abnormally low, and it is likely that most of the patients who were ventilated by the inexperienced medical students were in this situation. Of course, hyperventilation was better than hypoventilation under these conditions. A common observation in patients who are mechanically ventilated over long periods is that they complain of “air hunger” if the PCO2 is allowed to rise to near the normal level of 40 mmHg.
In 1953, there was another poliomyelitis epidemic, this time in Stockholm, and with the experience obtained in Copenhagen in the previous year, the management of patients with respiratory paralysis had improved. Nevertheless several pH values over 7.6 were reported in arterial blood [19].
A further fallout from these early measurements of pH, PCO2, and bicarbonate in blood were other indexes that improved our understanding of the respiratory and metabolic components of acid-base disturbances particularly when complicated mixed situations occurred. One measurement was the “standard bicarbonate,” which was the plasma bicarbonate concentration when the blood was exposed to a gas of normal PCO2 of 40 mmHg. This was often obtained by having the laboratory technician exhale over it! In effect, this measurement removed the respiratory component of the acid-base disturbance and allowed any metabolic compensation to be recognized. A similar concept had been suggested earlier by Van Slyke and also by Hasselbalch.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree