, Domenico Corrado2 and Cristina Basso1
(1)
Cardiovascular Pathology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
(2)
Cardiology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
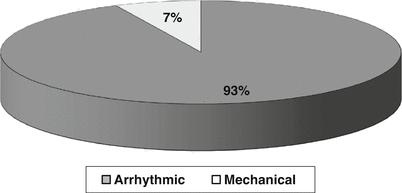
The Veneto Region is located in the Northeast of Italy with Venice as the capital. According to the Italian Census Bureau & Sports Medicine (1979–1999), the overall inhabitants were 437,900 – with the young population (12–35 years) of 1,386,650 and young athletes of 112,790 (M to F = 4:1) (Fig. 12.1). In this time interval, the cumulative incidence of SD was 1/100,000/year in young people aged less than 35 years old (SIDS excluded) [1–3]. Among nonathletes, the incidence was 0.9/100,000/year and among athletes was 2.3/100,000/year [1]. Thus the occurrence of SD was 2.5-fold in athletes vs. nonathletes (p <0.0001). In the time interval (1980–2013), a total of 650 SCD were studied at postmortem (201 F, 31 %): it was mechanical in 7 % and arrhythmic in 93 % [1] (Fig. 12.2).
Among the mechanical causes, pulmonary embolism accounted for 2 %, aortic rupture for 3 %, and others for 2 %. A congenital or genetic substrate of aortic rupture was always there: Marfan syndrome, bicuspid aortic valve, and aortic isthmic coarctation with or without bicuspid aortic valve. SCD occurred because of hemopericardium with cardiac tamponade, since the aortic dissection with external rupture always involved the intrapericardial ascending aorta (type A).
Coronary atherosclerosis was the cause of SCD in 18 % of cases, usually with a single obstructive atherosclerotic plaque located in proximal descending coronary artery (Fig. 12.3). Acute occlusive thrombosis was present only in 34 % in this group, mostly due to endothelial erosion [4].
In 2 % of cases, the acquired coronary artery disease was coronary dissection, usually in women [5].
Congenital coronary artery abnormalities were the culprit in 5 % of cases, mostly consisting of anomalous origin from the wrong sinus [6].
Myocarditis was diagnosed as the cause of death in 12 %. The inflammatory infiltrate was patchy. Molecular investigation, which was possible to be carried out also in paraffin-embedded myocardium, revealed coxsackievirus as the cardiotropic infective agent in half of the cases [7, 8].
Arrhythmogenic cardiomyopathy resulted to be the third cause accounting for 10 %. The fibrofatty replacement was frequently biventricular and even isolated in the left ventricle or segmental in the right ventricle. The presence of right ventricular aneurysms was a common finding, with the one located in the subtricuspid diaphragmatic region considered a pathognomonic marker of the disease [9, 10].
Hypertrophic cardiomyopathy, with asymmetric subaortic, midventricular, or apical hypertrophy, accounted for 9 %. Severe myocardial disarray and fibrotic scars, located within the asymmetric hypertrophy, were constant findings [11].
Mitral valve prolapse was the only structural disease in 8 % of subjects dying suddenly, largely prevalent in the female gender. In no case death was due to chordal rupture with pulmonary edema. Fibrosis of the papillary muscles and of the posterolateral free wall of the left ventricle was the most likely arrhythmogenic substrate. When available, the ECG disclosed polymorphic premature ventricular beats and repolarization abnormalities in the inferior leads [12].
Conduction system disease, investigated by serial sections technique, was the cause of SCD in 6 %. In the majority, it was an ECG-proven Wolff–Parkinson–White or Lown–Ganong–Levine ventricular preexcitation [13]. In the former, the accessory pathway was detected in the lateral, left or right, AV rings. In the latter, an atrio-hisian bypass bundle (“James” fascicle) or hypoplastic AV node was found at the septal AV junction. There was evidence of atrial myocarditis in half of cases. More rarely, a Lev–Lenegre disease with fibrosis of the branching His bundle was noted, probably explaining cardiac arrest through a sudden AV block.
Dilated cardiomyopathy was the cause of SCD in 4 % of cases. It presented with cardiomegaly with eccentric hypertrophy and biventricular dilatation, in the absence of any other explanation. Histology disclosed cardiomyocyte abnormalities (bizarre nuclei, perinuclear, halo) and patchy fibrosis in keeping with dilated cardiomyopathy [1, 7].
Congenital heart diseases were observed in 2 % of cases [14]. They included mostly postoperative tetralogy of Fallot, but also operated subjects for complete transposition by atrial switch. Eisenmenger syndrome along with natural history was also noted in a couple of cases.
Finally, SCD occurred in young subjects in whom the heart appeared normal at gross and histological examination (17 %) [1, 7, 8]. When ECG recordings were available, LQT, SQT, Brugada syndrome, and CPVT were clinically diagnosed. In the absence of ECG, we cannot exclude a concealed Wolff–Parkinson–White syndrome.
Molecular autopsy was employed in the recent years and allowed to reveal pathogenic gene mutations in cases of LQT, Brugada, and CPVT syndromes. Molecular genetic investigation of early cases, “left behind,” failed because of the impossibility to extract the DNA from FF-PET (see Chap. 11), confirming that the study should be performed in fresh or frozen preserved tissue [8].
As far as the athletes, arrhythmogenic cardiomyopathy accounted for 23 % of SCD victims, coronary atherosclerosis for 19 %, congenital coronary artery anomalies for 16 %, and hypertrophic cardiomyopathy for only 2 % [1–3, 15]. The discrepancies, when compared with the overall nonathlete population, have to be ascribed to the role played by effort in precipitating SCD [1] (Figs. 12.4 and 12.5). In fact, effort increases five times the risk of SCD in subjects affected by arrhythmogenic cardiomyopathy, because of stretching of the right ventricular free wall. In congenital coronary artery anomalies, the risk ratio between sedentary and sport activity is even higher, i.e. 1:80. By the way, while arrhythmogenic cardiomyopathy discloses subtle ECG abnormalities (T-wave inversion in right precordial leads) [16] (Fig. 12.6) coronary artery disease may exhibit a normal basal and stress ECG, as to escape pre-participation screening even when ECG is employed, like in Italy [3] (Figs. 12.7 and 12.8). On the opposite, the basal ECG is positive in 85 % of hypertrophic cardiomyopathy cases and, as such, allows to raise the suspicion of the disease, which is then confirmed at 2D echocardiography, which is the gold standard for diagnosis and is compulsory in our Country at pre-participation screening in case of doubt [17] (Fig. 12.9). Disqualification from sport activity is lifesaving, and this is the reason why in Italy, the rate of SCD in athletes due to hypertrophic cardiomyopathy is only 2 % vs. 26 % in the United States, a country where the pre-participation screening does not include the use of ECG [18–20]. In the Veneto Region, Northeast of Italy, in the time interval 1979–2004, the annual incidence of SCD declined by 89 % in screened athletes (P for trend <0.001). In contrast, the incidence of SCD did not demonstrate consistent changes over that time in unscreened nonathletes (Fig. 12.10).
12.1 Image Gallery
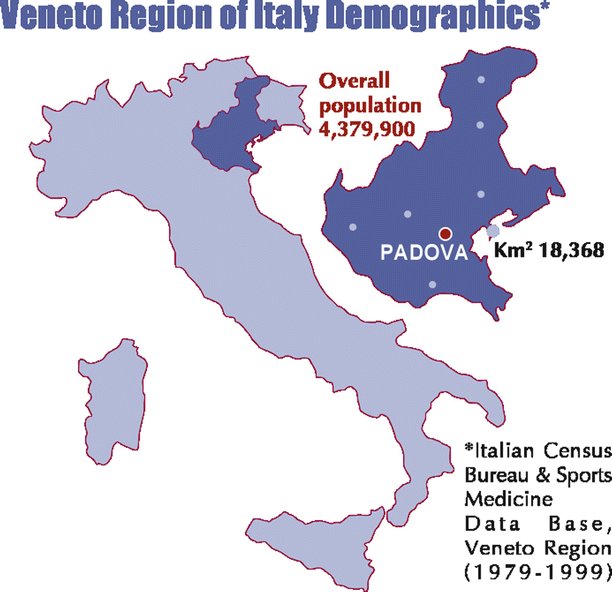
Fig. 12.1
The Veneto Region is located in Northeast Italy with an area of 18,368 km2. According to Italian Census Bureau & Sports Medicine database, in the time interval 1979–1999, a mean 4,379,900 overall population was calculated, including 1,386,650 young people and 112,790 young athletes (12–35 years old)
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


