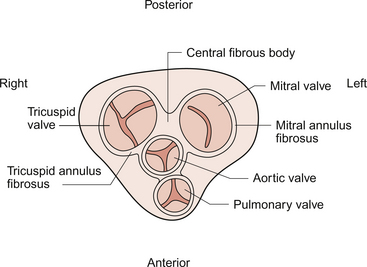
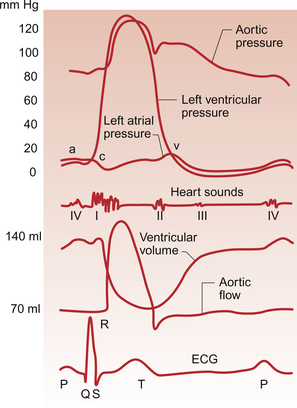
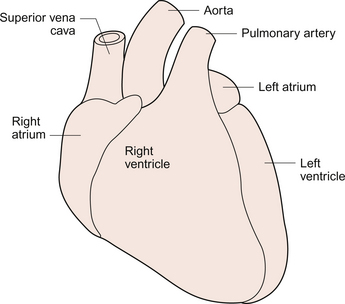
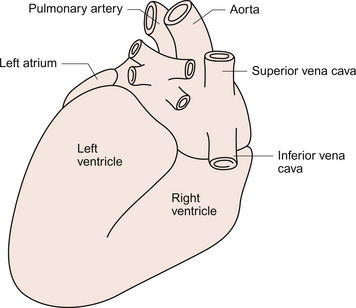
3 The heart lies obliquely in the thorax within the mediastinum—the space between the lungs which also contains the major blood vessels, oesophagus and trachea. It typically weighs about 325 ± 75 g in men and 275 ± 75 g in women. The heart can be described as having three surfaces and an apex. About two thirds of the heart is to the left of the mid-line. The anterior surface of the heart is formed mainly by the right ventricle and is in contact with the ribs and sternum (Fig. 3.1). The inferior surface of the heart is formed mainly by the left ventricle and is in contact with the diaphragm. The posterior surface of the heart is formed mainly by the left atrium. This surface is also known as the base of the heart. The apex which is anterior to the rest of the heart consists only of the left ventricle and forms an important clinical landmark when assessing the size of the heart. The aorta and the pulmonary trunk arise from the left and right ventricles respectively at the superior pole of the heart (Fig. 3.2). The superior and inferior vena cavae open into the upper and lower parts of the right atrium. There are four pulmonary veins which open into the back of the left atrium. The junction between the atria and the ventricles is marked by the atrioventricular groove and the junction between the ventricles both posteriorly and anteriorly is marked by the interventricular grooves. Fig. 3.1 External anatomy of the heart. Frontal (anterior) view of the external anatomy of the heart. Fig. 3.2 External anatomy of the heart. Posterior-inferior view of the external anatomy of the heart. The fibrous skeleton of the heart is a framework of dense collagen around the atrioventricular junction and the arterial outflow. It provides structural support for the heart, particularly by stabilizing the heart valves, and it also prevents the heart from being overstretched (Fig. 3.3). The electrical insulation provided by this fibrous skeleton forms the barrier between the atria and the ventricles and ensures that electrical conduction normally only occurs through the bundle of His (see Chapters 2 and 7). The heart is surrounded by the pericardium. The fibrous pericardium is a tough sac enclosing the heart and providing attachments to adjacent structures. The serous pericardium has a visceral layer which is attached to the surface of the heart and a parietal layer lining the fibrous pericardium. The smooth layers of the serous pericardium slide easily over each other and allow the heart to move within the thorax as it beats. The very narrow space between the layers of serous pericardium is filled with pericardial fluid. The right atrium has only a thin (about 2 mm) wall. There is a rough portion belonging to the auricular appendage and a smooth portion which receives the superior and inferior vena cava and the coronary sinus, the major part of the venous drainage for the heart itself. Blood flows from the right atrium into the right ventricle through an atrio-ventricular valve which has three cusps—the tricuspid valve (Fig. 3.3). The right ventricle is more muscular (about 4–5 mm thick) than the right atrium. The tricuspid valve is attached to the papillary muscles of the ventricular wall by the chordae tendinae (heart strings) which are made of fibrous tissue and help to stabilize the valve and prevent blood from flowing back into the right atrium when the ventricle contracts. The left atrium is thin walled (about 3 mm) and, like the right atrium, has a smooth and a roughened portion. The smooth-walled part is formed by the entrance of the four pulmonary veins. The atrioventricular opening in the left side of the heart is guarded by a valve shaped like a bishop’s mitre and formed from two cusps—the mitral valve. (In Shaw’s play, St Joan declares at her trial that ‘Bishops are never in the right’—this may help you to remember that the mitral valve is in the left side of the heart!). The left ventricle has the greatest wall thickness (8–15 mm) of all the cardiac chambers with internal muscular ridges throughout except for a small area at the aortic valve opening. At the tip of the apex the wall of the left ventricle is only about 2 mm thick. The mitral valve is anchored to the left ventricle by papillary muscles and chordae tendinae. The aortic valve has three semilunar cusps. There is a dilatation, the sinus of Valsalva, in the aortic wall just above each cusp. These dilatations allow blood to flow behind the cusps at the very beginning of ventricular relaxation in diastole and thus ensure that the aortic valve shuts and that the cusps are not flattened against the side of the aorta. They also allow blood to freely enter the right and left coronary arteries (see Chapter 5). The arterial blood supply of the heart is via the right and left coronary arteries which originate from the aorta near the aortic valve (see Chapter 5). The left coronary artery branches into the left circumflex and left anterior descending arteries. The right coronary artery branches into the marginal and right posterior descending arteries. The venous drainage of the heart is via a number of veins which drain into the coronary sinus on the inferior surface of the heart in the atrioventricular sulcus. The coronary sinus drains into the right atrium. The events which comprise the cardiac cycle on the left side of the heart are shown in Figure 3.4. The equivalent values for the right side of the heart are qualitatively similar but the pressures are lower since the pulmonary circulation is a low-resistance circu lation. The onset of ventricular systole is indicated by the first heart sound which is produced by closure of the atrioventricular valves. The end of ventricular systole is indicated by the second heart sound which is produced by closure of the aortic and pulmonary valves. On the ECG the P wave corresponds to atrial depolarization (see Chapter 7) and thus marks the onset of atrial systole. The QRS complex is associated with ventricular depolarization and thus the onset of ventricular systole. The functioning of the heart as an effective pump depends upon the valves between the atria and the ventricles (the tricuspid and mitral valves) and those between the ventricles and the main distributing arteries (the aortic and pulmonary valves) functioning correctly. In Figure 3.4 the left ventricular pressure wave begins with a small surge of pressure when the left atrium contracts and ‘top ups’ the ventricle. In some individuals with a hypertrophied left atrium pumping blood into a stiffened left ventricle, as may occur in ischaemic heart disease or hypertension, a sound is generated which is referred to as the fourth heart sound. The increase in ventricular volume associated with atrial systole is very small as most ventricular filling is passive and occurs in the early stage of diastole when blood flows through the relaxed atrium into the relaxed ventricle. With the onset of ventricular contraction ventricular pressure rises and the mitral valve closes. This, along with the normally concurrent closure of the tricuspid valve, causes the first heart sound. If venous return is increased acutely then the closure of the tricuspid valve in the right side of the heart may be slightly delayed and the first heart sound will be ‘split’, that is closure of the two valves will be heard separately. If either valve fails to close fully then the murmur of mitral or tricuspid regurgitation, an early systolic murmur, will be heard. The ventricle empties the stroke volume of blood into the aorta (see Chapter 4) and ventricular volume typically falls by about 70 mL (Fig. 3.4). Ventricular and aortic pressures follow a similar path as the contraction of the ventricles opposes the elastic recoil of the aorta. Blood flow in the aorta rises substantially at this time. As ventricular relaxation proceeds, ventricular pressure falls and when it is below the pressure in the aorta the aortic valve closes. This is helped by a backflow of blood created by the sinus of Valsalva as described above. Closure of the valve creates a short-lived dip in aortic pressure called the dicrotic notch. The closure of the aortic and pulmonary valves generates the second heart sound. If either the aortic or pulmonary valve fails to close fully then the murmur of aortic or pulmonary regurgitation may be heard. This is an early diastolic murmur. Ventricular pressure continues to fall and eventually the pressure in the ventricle is less than that in the atrium. At this point the mitral valve opens and blood flows into the ventricle (Fig. 3.4). If the mitral or tricuspid valve fails to open properly then the early diastolic murmur of mitral/tricuspid stenosis may be heard. Opening of these valves between the atria and ventricles heralds the period of rapid ventricular filling and may occasionally, especially in individuals with low heart rates and high stroke volumes, produce a third heart sound which is said to be analogous to a ‘sail flapping in the wind’. After closure of the aortic valve pressure in the aorta and thus blood flow is maintained by the elastic recoil of the artery wall. The oscillations in aortic pressure are therefore not as large as those seen in the ventricle and aortic flow, although it is pulsatile, is much more uniform than it would otherwise be. This is important to ensure continued perfusion of all the tissues of the body during diastole (see Chapter 10). The pressure wave in the left atrium is shown in Figure 3.4. The right atrial pulse is qualitatively similar and, since there are no valves at the entrances to the atria, a similar pulse may be seen in the jugular vein if the patient’s head and thorax are tilted back at about 45 degrees. The ‘a’ wave of atrial pressure (7 mm Hg in the left atrium and 5 mm Hg in the right atrium) corresponds to atrial systole, the ‘c’ wave corresponds to the closure of the atrioventricular valves which bulge back into the atria as they close. The ‘v’ wave of the jugular or atrial pulse results from atrial filling and immediately precedes the opening of the tricuspid and mitral valves. The case history of a young girl with a suspected cardiac valve problem is introduced in Case 3.1:1.
THE HEART AS A PUMP
VALVE FUNCTION AND VALVE DISEASE
Functional anatomy of the heart
The cardiac cycle
Thoracic Key
Fastest Thoracic Insight Engine