Baseline to year 2
P value
Year 2 to year 4
P value
Year 4 to year 6
P value
Characteristic
Subjects without diabetes mellitus at the beginning of the interval
6,486
4,076
753
Randomized treatment
ACE inhibitor vs. diuretic
0.550 (0.431–0.704)
<0.001
0.816 (0.586–1.138)
0.23
0.863 (0.403–1.851)
0.71
CCB vs. diuretic
0.727 (0.579–0.913)
0.006
0.0740 (0.536–1.023)
0.07
0.955 (0.579–1.902)
0.90
It is believed that glucose abnormalities in association with thiazide diuretic use are through loss of urinary potassium with consequent diminished serum potassium levels [22]. The latter inhibits or impairs the release of insulin from the pancreatic beta cells. It is also hypothesized that diuresis can lead to decreased blood volume and cardiac output, which activates the sympathetic nervous system, leading to reduced blood flow to the skeletal muscle and ultimately to peripheral insulin resistance [23] (Fig. 44.1).
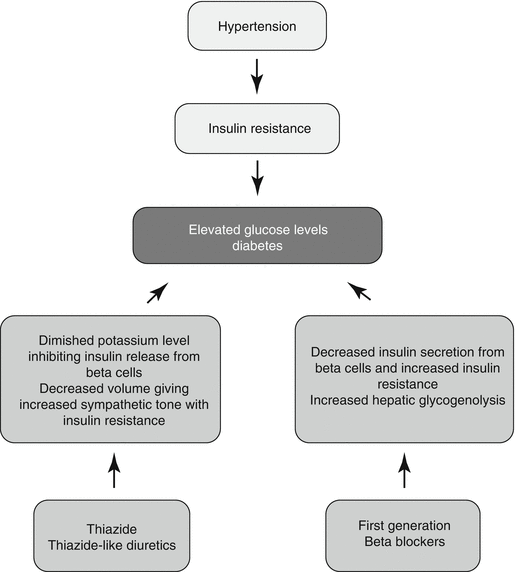

Fig. 44.1
Proposed diabetogenic mechanisms of thiazide and thiazide-like diuretics and first-generation beta-blockers
To gain insight into these concepts, we examined the Antihypertensive Lipid-Lowering Heart Attack Prevention Trial (ALLHAT) study database. ALLHAT was a large (>42,000 participants) multicenter randomized clinical trial designed to determine whether the occurrence of fatal CHD or nonfatal myocardial infarction would be lower for high-risk patients with HTN assigned to first-step therapy with the calcium channel blocker (CCB) amlodipine (Chap. 37), the ACEI lisinopril, or the α-adrenergic receptor blocker doxazosin compared to the thiazide-type diuretic chlorthalidone during 5 years of treatment [24]. The doxazosin arm was discontinued after only 3.3 years because of futility of finding a significant benefit for CHD incidence and also because doxazosin was associated with a significantly higher incidence of CVD and especially heart failure as compared to chlorthalidone [25]. Following the full 5 years of treatment, there were no differences in the incidence of the primary outcome (fatal CHD and nonfatal MI) or all-cause mortality for amlodipine or lisinopril as compared to chlorthalidone. However, the thiazide-based treatment resulted in a clinically important significantly lower risk of heart failure [3].
We conducted a post hoc analysis of ALLHAT to investigate differences in elevated FG levels, change in FG levels, and incident diabetes mellitus in the three treatment groups. Unlike previous studies, we were able to examine the impact of any glucose changes associated with treatment assignment to the risk of CVD and renal disease in participants without diabetes at baseline. By design, there was little overlap in the use of the three classes of antihypertensive medications so valid comparisons could be made. In addition, the number of participants and events in each arm of the study was sufficiently large to provide adequate statistical power.
In the first part of our analysis [26], we found that FG levels increased steadily during a mean follow-up of 4.9 years, regardless of the antihypertensive therapy assignment. Participants randomized to treatment with chlorthalidone had a significantly higher level of FG and a higher incidence of new-onset diabetes mellitus at years 2 and 4 of follow-up compared with those randomized to lisinopril or amlodipine treatment. However, the differences in mean FG level change between the treatment groups were small (3.0 and 1.5 mg/dL between chlorthalidone and amlodipine and 5.0 and 4.0 mg/dL between chlorthalidone and lisinopril at years 2 and 4, respectively). Further analyses found little association between serum potassium levels or changes in serum potassium levels and FG elevations in those taking chlorthalidone.
These small differences in FG levels translated into higher odds of new-onset diabetes mellitus (FG > 125 mg/dL) at 2 years in those taking chlorthalidone compared with those taking amlodipine or lisinopril. In other words, a small change in FG levels moved many participants over the 125 mg/dL cutoff point used to define diabetes mellitus. During the first 2 years, the odds for developing new-onset diabetes were lower for those assigned to lisinopril (odds ratio [OR], 0.55 [95 % confidence interval [CI], 0.43–0.70]) or amlodipine (OR, 0.73 [95 % CI, 0.58–0.91]) compared to their counterparts assigned to chlorthalidone. At years 4 and 6, assignment to amlodipine or lisinopril was still associated with a lower OR of developing new-onset diabetes, but the ORs were no longer statistically significant (Table 44.1).
The 4- and 6-year cumulative incidence of new-onset diabetes among participants without diabetes at baseline was 11.0 and 13.8 % in those assigned to chlorthalidone, 9.3 and 12.0 % in those assigned to amlodipine, and 7.8 and 11.0 % in those assigned to lisinopril. These rates allow for a calculation that provides an important perspective on thiazide-associated diabetes. If amlodipine is assumed to be metabolically neutral, then (based on the 4-year rates) 85 % (9.3/11.0) of the new-onset diabetes associated with a thiazide diuretic is not “causally related” to the use of a thiazide diuretic, at least in an older, mostly overweight, ethnically diverse population.
Findings similar to these have been reported in two other studies that reported on change in FG levels with antihypertensive medications [27, 28]. In both these studies, the changes in FG levels were small (mean, 2.0–3.5 mg/dL), but the relative risk of developing new-onset diabetes was significantly increased.
We next examined whether these differences in FG impacted CVD and renal disease outcomes. We failed to find an effect of the changes in FG level on any study end point, both for an exploration of all the treatment groups combined and for the chlorthalidone group separately. Further, the hazard ratio for a 10 mg/dL change in FG level was no larger for chlorthalidone than for amlodipine or lisinopril. For incident diabetes mellitus, CHD was the only outcome with a statistically significant increased hazard ratio, and this did not differ significantly across the three treatment groups (Fig. 44.2).
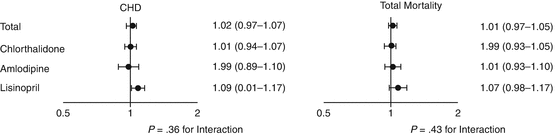

Fig. 44.2
Cox regression models showing the hazard ratio and 95 % confidence intervals associated with a 10 mg/dL (0.56 mmol/L) increase in fasting glucose during the first 2 years of follow-up for subsequent CHD and total mortality (Based on Ref. [26]. Reproduced with permission from the American Medical Association)
These ALLHAT findings are consistent with other studies. In a 14-year follow-up of the Systolic Hypertension in the Elderly Program [27], diabetes mellitus diagnosed during chlorthalidone therapy was not associated with a significant increase in CVD mortality (HR, 1.04 [95 % CI, 0.75–1.46]) when compared with diabetes mellitus that occurred in the absence of diuretic administration, which was associated with a significant increase in CVD mortality (HR, 1.56 [95 % CI, 1.12–2.18]). In a second study, a 15-year follow-up of 686 middle-aged adults with HTN treated with diuretics [28], incident diabetes mellitus did not have a significant effect on CVD mortality, whereas prevalent diabetes mellitus at baseline did.
An explanation can be proposed as to why increased blood glucose and diabetes mellitus in association with thiazide-like diuretic treatment did not lead to an increase in CVD risk. Previous studies have shown that glucose levels decrease when diuretic therapy is discontinued [22]. In contrast, “classic” diabetes mellitus is characterized by persistent elevation of blood glucose levels. This suggests that the elevation in glucose levels, which can occur during diuretic therapy, is likely to have been caused by mechanisms other than insulin resistance – the underlying pathway for “classic” diabetes mellitus. As discussed above, only a small proportion of the cases of diabetes mellitus that arises during diuretic therapy actually appears to be “causally related” to the diuretic. On the other hand, incident diabetes mellitus in participants treated with lisinopril was associated with a significantly high risk of CHD. Also among those treated with amlodipine, patients who developed diabetes had a higher total mortality and stroke. Given that these medications are “glucose protective” or “glucose neutral,” respectively, the development of diabetes in association with their use suggests the presence of a high degree of insulin resistance. It should also be remembered that a rise in FG levels associated with thiazide diuretics occurs most often in people who would probably have developed diabetes mellitus anyway (insulin resistant, overweight, or obese) over time.
In a subsequent analysis [29], we employed administrative data sets to explore the impact of antihypertensive medications on glucose metabolism in the ALLHAT participants during 4–5 years of observation after completion of the trial. During the period of posttrial observation, the ALLHAT participants were no longer required to continue their assigned first-step study medication. We conducted this analysis because it could be argued that the mean follow-up period of 4.9 years during the trial might be insufficient to detect the detrimental effects of elevated glucose levels on CVD outcomes. An increase in blood glucose might lead to an increase in CVD risk only over a longer period of time. Posttrial monitoring of participants in the UK Prospective Diabetes Study (UKPDS) [30] suggested that a sustained period of glycemic control in patients with newly diagnosed diabetes mellitus reduced cardiovascular morbidity and mortality, but two decades or more of follow-up were required before the effects achieved statistical significance. The converse could be true for recognition of new-onset diabetes as an adverse effect of antihypertensive therapy.
In our analysis of health experience during extended follow-up of the ALLHAT participants, those with diabetes mellitus at baseline experienced higher rates of CVD as compared to their counterparts without diabetes mellitus. Those with incident diabetes generally had outcome rates that were intermediate between those with and without baseline diabetes. A more provocative finding was the comparison between those who did and those who did not develop diabetes during the trial, based on treatment assignment. The HRs in the chlorthalidone cohort with versus without incident diabetes for CVD mortality (HR, 1.04 [95 % CI, 0.74–1.47]), all-cause mortality (HR, 1.04 [95 % CI, 0.82–1.30]), and non-CVD mortality (HR, 1.05 [95 % CI, 0.77–1.42]) were consistently lower than the comparable HRs in the corresponding amlodipine and lisinopril cohorts (HRs, 1.22–1.53). For example, those who developed diabetes in the chlorthalidone cohort had a statistically insignificant increase in the risk for total CHD events when compared with those who remained nondiabetic (HR, 1.18 [95 % CI, 0.77–1.81]), whereas the opposite was true for participants with versus without incident diabetes who had originally been assigned to lisinopril (HR, 2.57 [95 % CI, 1.45–4.54]). Similarly, the HRs (CIs) for all-cause mortality and stroke were not significantly different in the chlorthalidone cohort based on incident diabetes status: 1.04 (0.82–1.30) and 0.91 (0.49–1.67), respectively. Thus, the results were similar to and confirmed the results of the first-phase (in-trial) period of ALLHAT.
In summary, the treatment experience in ALLHAT and other studies suggests that low-dose thiazide and thiazide-like diuretics are associated with small increases in FG levels as compared to the ACEI lisinopril and the CCB amlodipine. These differences diminish over time. The magnitude of the FG difference does not appear to result in a clinically significant increase in CVD risk.
44.3 Renin-Angiotensin System Blockade: ACEIs and ARBs
Upregulated bradykinin and nitric oxide, both of which promote increased skeletal muscle and pancreatic blood flow, are suggested to be the mediators of the improved glycemia noted during ACEI and ARB therapy [31]. Use of agents from these drug classes is also reported to improve angiotensin-II-mediated oxidative stress in the beta cell [32]. On account of their favorable glucose effect, ACEIs and ARBs are considered the “preferred” antihypertensive medications in people at high CVD risk (Chap. 36).
In the preceding section, we showed that lisinopril was associated with a slightly lower FG level as compared to the thiazide-like diuretic chlorthalidone with little or no clinical impact. The next question we address is whether RAS blockade prevents new-onset glucose disorders or ameliorates prevalent glucose disorders better than placebos.
In the Heart Outcomes Prevention Evaluation (HOPE) trial [33], the risk of self-reported incident diabetes was less in those assigned to the ACEI ramipril compared to placebo (3.6 % vs. 5.4 %; relative ratio [RR] 0.66 [95 % CI 0.51–0.85]; P < 0.001). In the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial [34], the risk of incident diabetes was less in those assigned to the ARB valsartan compared to placebo (33.1 % vs. 36.8 %; RR 0.86 [0.80–0.92]; P < 0.001). In contrast, treatment with ramipril did not reduce incident diabetes in people with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (17.1 % vs. 18.5 %; RR 0.91 [0.80–1.03]; P = 0.15) in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial [35]. This study was specifically designed to investigate the effects of RAS blockade on diabetes prevention. The Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial [36] also found no evidence that the addition of telmisartan to usual care prevented incident diabetes or led to regression of IFG or IGT in persons at high risk for cardiovascular disease but free from diabetes. During a 56-month median period of treatment, 21.8 % of the participants treated with telmisartan compared to 22.4 % of their counterparts on placebo developed diabetes (RR 0.95 [95 % CI 0.83–1.10]; P = 0.51). Participants with IFG and/or IGT at baseline were equally likely to regress to normoglycemia (26.9 % vs. 24.5 %) or to progress to incident diabetes (20.1 % vs. 21.1 %; P = 0.59) during telmisartan or placebo therapy.
Variation in patient age and prior history of CVD may have played important roles in explaining study outcome differences. The NAVIGATOR trial (which used an ARB) enrolled individuals who were younger than those in TRANSCEND (which also used an ARB) and had a much lower prevalence of CVD (24 % vs. 80 %). Increasing age and prevalent CVD are associated with increased insulin resistance [37, 38].
Many of the ACEI studies were post hoc analyses in which incident diabetes was not a prespecified outcome. The results relied on physician-reported or self-reported incident diabetes rather than measures of FG or 2-h post-challenge levels of blood sugar. This makes it likely that many of the study participants with seemingly normal glucose metabolism would have been diagnosed with new-onset diabetes had they been thoroughly investigated.
In summary, there is no strong evidence that RAS blockade has an important antidiabetic effect when added to other cardiovascular agents for the treatment or prevention of atherosclerotic heart disease (ASHD) in people at high risk, most of whom have HTN.
44.4 First-Generation Beta-Blockers
First-generation β-blockers, such as propranolol and metoprolol, have been associated with worsening of glycemia and an increased risk of diabetes mellitus. The possible mechanisms include inhibition of pancreatic insulin secretion through nonselective blockade of β2-adrenergic receptors, inhibition of peripheral glucose uptake, and resultant unopposed α2-adrenergic receptor-mediated stimulation of hepatic glycogenolysis [39] (Fig. 44.1). Newer-generation beta-blockers, such as carvedilol and nebivolol, have fewer metabolic effects than first-generation beta-blockers and are considered metabolically favorable [40].
Despite their glycemic effects, first-generation beta-blockers such as propranolol, metoprolol, and atenolol are still widely used. They are effective for HTN management and for preventing ischemic cardiovascular disease and sudden death. In the UK Prospective Diabetes Study [41], atenolol-based therapy was as effective in cardiovascular protection as was ACE-based therapy. In the Beta-Blocker Heart Attack Trial [17], a first-generation beta-blocker (propranolol) was effective for preventing recurrence of ischemia compared to a placebo in people with diabetes mellitus. In other studies, diabetic people prescribed first-generation β-blockers had significantly lower 1-year mortality rates than those not receiving these agents, regardless of type and severity of diabetes mellitus [18, 19].
In the Atherosclerosis Risk in Communities (ARIC) study [42], an observational cohort, there was an increase in the risk of diabetes associated with the use of beta-blockers (RR, 1.28; 95 % CI, 1.04–1.57). Similar findings have been noted in other observational studies [43, 44], In contrast, a retrospective, observational cohort study [45] of previously untreated patients, aged ≥66 years, identified as new users of an antihypertensive drug class, found that neither ACE inhibitor use (HR 0.96 [95 % CI 0.84–1.1]) nor beta-blocker use (0.86 [0.74–1.0]) was associated with a statistically significant difference in type 2 diabetes mellitus incidence when compared with the CCB control group (n = 76,176). In the secondary analysis (n = 100,653), compared with CCB users, T2DM incidence was not significantly different between users of ACE inhibitors (0.97 [0.83–1.1]), beta-blockers (0.84 [0.7–1.0]), or thiazide diuretics (1.0 [0.89–1.2]).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


