Knowledge gap
Strategy
Example of present or future state
Unifying theory of PH across WHO groups
Cross-sectional deep phenotyping; consensus phenotypic definitions
NHLBI PVD phenomics; consensus documents [1]
Predicting PAH disease progression
Longitudinal deep phenotyping
Serial MRI as predictor of outcome [2]
Serial biomarkers
Serial BNP or NTproBNP
Markers of progression of vascular remodeling and proliferation
PBMC and Serum OMIC signatures correlated with serial hemodynamics, outcome
Serial OMICS in clinical trials of therapeutic agents that include serial hemodynamics and outcome measures
Lung vascular imaging
Lung FDG uptake [3]
Genetics of PH
GWAS; Exome sequencing
PAH susceptibility loci [4]
Pathobiology of PH
Network biology
MicroRNA-21 [5]
Explanted lung tissue/serum analyses
Myocardial metabolism
PET
Peripheral muscle factors
MR spectroscopy; cardiopulmonary rehab
Best RV function measures
RV imaging
RV-PA coupling
Simplification of method
Exercise hemodynamics
Standardization, reporting of experience, relation to outcome
Optimizing clinical trial recruitment in PAH
Understanding barriers, trial design, cultivating culture of participation
Patient interviews [36], Advocacy, Central repository for research options e.g., Clinicaltrials.gov, minimizing patient burden in trials
Therapies thus far available for pulmonary hypertension have focused on vasodilation, despite the fact that the more severe forms of pulmonary hypertension are inevitably accompanied by vascular remodeling that results in reduction of the cross-sectional area of the pulmonary vascular bed. The subsequent development of right heart failure is usually ultimately responsible for pulmonary arterial hypertension (PAH)-related mortality. Non-vasodilator therapies could either target the pulmonary vascular remodeling process, the right ventricular response to the adverse pulmonary hypertension milieu, or both. Full restoration of normal pulmonary vascular resistance is not necessary; achieving a state in which the right ventricle (RV) is adequately coupled to the pulmonary vascular load, and preventing progression of RV dysfunction and the pulmonary vascular load would be adequate.
The Future of Clinical Trial Design
The move away from a vasodilator-centric view to approaches that target pulmonary vascular remodeling and/or the RV, combined with a wide array of possible therapeutic targets, creates several issues from a clinical trial perspective.
1.
Trial organization and funding
2.
Trial duration
3.
Surrogate endpoints
4.
Definitive endpoints
5.
Patient selection
6.
Patient recruitment strategies
Trial Organization and Funding
A July 2014 meeting in Bethesda, Maryland was inspired by the frustration of the pulmonary hypertension research community at the slow pace of development of industry sponsored research in the non-vasodilator PH arena. This meeting brought together clinicians, clinical trialists, bench researchers, representatives of National Heart, Lung, and Blood Institute (NHLBI), and industry. The goal of this meeting was to showcase the range of most promising basic research and early translational data, learn from recent missteps, and set the framework for future innovative and collaborative approaches. Traditionally, most pulmonary hypertension drug trials have been industry initiated and funded. Future advances in pulmonary hypertension therapy may either result from repurposing of agents from other fields such as Oncology, or specific PH drug development within industry or academia. If already available compounds are either off patent or nearly so, then the interest of industry in developing them for PH is very limited and unlikely to occur if the PH community relies on industry alone to drive the funding. A classic and unfortunate recent example is that of imatinib for PAH indications. In the IMPRES study, imatinib was associated with a highly significant improvement in the primary endpoint of 6 min walk distance, despite most of the patients being on double or triple drug therapy that often included prostanoids [37]. The incidence of cerebral hemorrhage was higher in the treatment arm, with all such events occurring in patients also on warfarin. In addition, the time to clinical worsening endpoint trended in the wrong direction, reflecting an increased hazard of early hospitalization in the treatment arm. This was attributed to imatinib-related fluid retention, although this could not be distinguished readily from PAH disease progression. In retrospect, a higher awareness of this issue could have allowed a contemporaneous diuretic approach analogous to that often employed during initiation of beta-blocker therapy in left heart failure. Future trials of repurposed agents must carefully consider known side effect profiles and be proactive about expected response to their development. In addition, the dropout rate in the imatinib treatment arm was high, and those patients were not subsequently followed for outcome. Future trials must make every effort to continue to collect data on patients that are withdrawn or who drop out, albeit recognizing that doing so can be logistically challenging. Discussing the importance of such follow up with patients a priori may enhance their willingness to continue to be followed for purposes of optimizing trial integrity, and should be a part of future trial design. Avoidance of clinical worsening endpoints that include components indistinguishable from disease progression must also be a key component of future trial design. Imatinib may have been further developed for PAH if it had not been close to going off patent, reducing interest of the pharmaceutical industry in further pursuing the concept.
In the future, academically driven consortiums will play an increasingly important role in drug development and clinical trial completion in pulmonary hypertension. Models for such consortiums are available, including the Eastern Cooperative Oncology Group and the Acute Respiratory Distress Syndrome Network. The National Heart, Lung, and Blood Institute of the National Institutes of Medicine (NHLBI/NIH) models include the highly successful Heart Failure Research Network that, for example, completed the landmark RELAX trial studying sildenafil in the treatment of heart failure with preserved ejection fraction [38]. The successful NHLBI funded Pulmonary Arterial Hypertension Biorepository, spearheaded by Nichols at the University of Cincinnati Childrens Hospital, demonstrates the ability to pool biological and phenotypic resources that will help to speed the therapeutic discovery process. Although the REVEAL registry of PAH is industry funded, the steering committee is fully academic, and the extensive epidemiologic observations of that study demonstrate the value of a large number of PH centers contributing routinely obtained clinical phenotyping toward a common set of goals [39–58]. The recent NHLBI funded development of a consortium of pulmonary hypertension centers that will carefully phenotype a large cohort of patients with pulmonary hypertension across World Health Organization (WHO) Groups I, II, III and IV will include detailed genomic, proteomic and metabolomic characterization that promises to identify homologies that unite this seemingly disparate set of disorders. (RFA – HL-14-027: Redefining Pulmonary Hypertension Through Pulmonary Vascular Disease Phenomics [PVDOMICS]; nhlbi.nih.gov). The potential for a comprehensive “omics” approach is made possible by remarkable advances in high throughput technology, bioinformatics, and network biology. The discovery of microRNA-21 as a potentially important factor in pulmonary vascular disease represents an exciting example of such a novel approach [5, 59]. This effort promises to be complemented by the recent NHLBI decision to fund a network of centers to carry out multicenter trials in patients with pulmonary hypertension and also in patients with parenchymal lung disease. The visionary decision of the Pulmonary Hypertension Association to fund an additional center for the PVDOMICS network illustrates what it is hoped will be a burgeoning trend toward collaborative research approaches that may include academic, public nonprofit/charitable organizations, private funding sources, and/or pharmaceutical industry collaboration. An additional example of this exciting trend is the NHLBI VITA initiative (Vascular Interventions/Innovations and Therapeutic Advances Program). (http://www.nhlbi.nih.gov/research/resources/vita.htm).
Trial Duration
The duration of therapy necessary to achieve a clinical endpoint depends upon the nature of that endpoint, the severity of illness of the population, their rate of disease progression, and whether they are already receiving reasonably effective therapy. It also depends upon the mode of action of the agent being studied, and the tolerability of that agent, which impacts the frequency of study drug discontinuation. The required therapy duration has increased steadily (e.g., 12 weeks to show a mortality benefit for epoprostenol or an improvement in 6 min walk with bosentan, vs. the 6 month placebo controlled phase for the imatinib study). Furthermore, the number of patients required to achieve adequate power, the length of time necessary to recruit them, and the number of centers that must be involved, have all increased and likely will continue to do so.
Surrogate Endpoints
“A surrogate does not an endpoint make” is the mantra of the clinical trialist when warning about the pitfalls of trial design [60]. Yet there is a tension between the need for properly conducted clinical trials with robust endpoints, and the difficulty of studying a rare disease that already has a host of (albeit ultimately inadequate) treatments available. This tension is exacerbated by the growing number of therapeutic targets and difficulty in establishing priorities. Surrogate endpoints are often useful in phase I and II studies in order to obtain early signals of biologic effect and efficacy. For vasodilator agents, acute changes in hemodynamics serve such a purpose. For pro-apoptotic and anti-proliferative agents, such straight forward surrogates are lacking. Accordingly, there is an unmet need for advances in the capacity to detect such effects prior to the point at which hemodynamic or clinical consequences are detectable. Systematic serial blood draws for biomarkers of response (known and to be discovered) should be a fundamental component of trials of anti-proliferatives in the future. Successful identification of changes in biomarkers that track with subsequent clinical response could help not only to sort which agents are most promising, but may also help to identify which patients are in fact responding to the agent. Baseline testing may also identify which patients are likely to respond, based upon genetic characteristics or other “omic” signatures. This “personalized medicine” approach has been particularly useful in cancer research and treatment. Tumor shrinkage is such a terrific surrogate for clinical response, that a study of a new cancer therapy can identify a subgroup of patients who respond, even if it is only 20 % of the studied population. If it were necessary as an early signal of efficacy to study every cancer therapy based upon survival of a whole treated cohort, many treatments that are useful only in a subset of patients would never have been identified as effective, since the power to detect clinical benefit in the entire cohort would be low. Consider the possibility that imatinib has a striking impact on outcome of 20 % of treated patients. Clear proof of that impact is difficult to achieve when there were many other patients who either did not tolerate the therapy or were non-responders. Hemodynamic effect is a tempting (albeit imperfect) surrogate in such a situation, but without a better methodology (e.g. biomarker of proliferative milieu) to identify a priori those probable responders, designing a trial that would recruit only likely responders, and then permit judicious application of the therapy, is difficult.
Definitive Endpoints
In the future, some clinical trials in PAH will utilize survival as the primary endpoint. Survival will be a primary endpoint because in the world of anti-proliferatives, a highly effective agent will have an impact on survival in an advanced PAH population within a feasible time frame and in a patient population of feasible size. Furthermore, one or more of such trials will be positive within the next decade. Is this a bold prediction? Perhaps, but it is not at all beyond the realm of possibility. At some point, a bold advancement will occur, revolutionizing the treatment of PAH. Consider the quandary of restenosis after coronary angioplasty. That recalcitrant problem was the Achilles heel of interventional cardiology for many years, and many compounds were tested without success. Then one day the sirolimus eluting stent was developed, and the world tilted.
The same will happen in PAH. How soon it will occur depends upon the success of the PAH community in fostering research, the degree of innovative thinking regarding trial design and drug discovery, the level of interest of scientists and clinicians in pushing the field, and the willingness of PAH patients to participate in clinical trials. Perhaps there is also an element of serendipity and luck. But as the old saying goes: “I am a great believer in luck. The harder I work, the more of it I seem to have.”
In the future, the PAH community will continue to work hard, creating their own luck. Hospitalization as a critical component of a composite endpoint has recently thrice been a major driver of a positive outcome in PAH trials including the macitentan pivotal trial [61]. Two additional phase 3 studies achieving such an endpoint are not yet published as of this writing: AMBITION (AMBrIsentan and Tadalafil in patients with pulmonary arterial hypertensION) (up front combined ambrisentan and tadalafil) and GRIPHON (Prostacyclin (PGI2) Receptor agonist In Pulmonary arterial HypertensiON) study of the IP receptor agonist selexipag (phase 2 reference [62]). The expense and effort to successfully enroll and complete these studies represent landmark clinical trial efforts in PAH.
Patient Selection
Clinical trials will increasingly need to focus on those patients at significant risk of adverse outcome within a year. The tendency in clinical trials has been to recruit patients that have long durations of disease and are therefore proven survivors. If that is combined with quite compensated clinical status, the trials are unlikely to meet efficacy endpoints within a set trial duration, and event driven trials will take too long to achieve an adequate number of events. Furthermore, patients who are fairly well compensated have little interest in trial participation.
Patient Recruitment Strategies
Trials will increasingly permit patients to be on a wide variety and number of PAH therapies, including parenteral prostanoids, since disease modifying agents will need to have demonstrable effects regardless of the background vasodilator therapy. This will make it easier to identify eligible patients in countries that have wide access to PAH therapies. If such patients are also at a point in their disease trajectory where they are seeking opportunity, then they will be more frequently willing to participate. This should facilitate trial launch, monitoring, and successful completion.
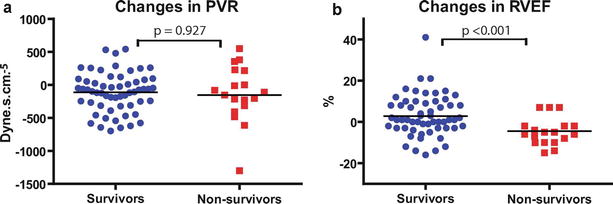
Assessment of Right Ventricular Function, Coupling, and Reserve
Quantitation of right ventricular function has historically been difficult, reflecting failure to perceive its importance in an LV-centric world, unusual geometry, and limitations in adequate visualization. As well discussed in earlier chapters, great strides have been made, including new echocardiographic tools such as strain, improve MRI techniques, sophisticated pressure volume loop analysis, and greater understanding of how changes in RV function over time correlate with clinical outcomes. The concept of coupling of the RV to the pulmonary circulation has also shed light. Measures of right ventricular function will increasingly play a role as exploratory endpoints of novel therapies and as secondary endpoints in phase III studies. From a clinical perspective, measurement of RV function will continue to gain acceptance as a critical component of longitudinal care. Recognition of the relationship between right ventricular function and outcome has become increasingly clear (Fig. 23.1). A desirable further development would be the creation of clear measures of right ventricular reserve. During exertion, cardiac output must increase in order to meet the needs of working muscle. Limitations in cardiac output response can manifest as dyspnea, lightheadedness, hypotension, and syncope. Noninvasive cardiopulmonary exercise testing can detect limitation in cardiac output response as manifested by a plateau in oxygen consumption, while invasive exercise testing can measure not only cardiac output, but also right atrial and pulmonary artery pressures. If the right heart is unable to maintain compensation during exercise, the right atrial pressure may rise, PA pressure may rise and then start to fall, and cardiac output fails to augment appropriately. The relative merits of 6 min walk testing, noninvasive and invasive cardiopulmonary exercise testing in the assessment of adequacy of therapy remain under explored and should be studied further.