General Classification of Supraventricular Tachyarrhythmias
As described in Chapter 2, most supraventricular tachyarrhythmias are caused by either abnormal automaticity or reentry.
Automatic Supraventricular Tachyarrhythmias
Automatic supraventricular tachyarrhythmias are relatively uncommon, except in acutely ill patients. When they do occur, they are usually associated with fairly obvious metabolic disturbances, the most common being myocardial ischemia, acute exacerbations of chronic lung disease, acute alcohol ingestion, and electrolyte disturbances. The arrhythmias usually arise from ectopic automatic foci located somewhere within the atrial myocardium. Characteristically, automatic atrial tachycardias display the typical warm-up phenomenon seen with automatic rhythms (i.e. the rate accelerates after its initiation). The peak rate is usually less than 200 beats/min. There is a discrete P wave before each QRS complex, and the P wave configuration generally differs from the sinus P wave (depending on the location of the automatic focus within the atria). Because the AV node is not necessary for the maintenance of the arrhythmia, AV block is commonly seen during automatic atrial tachycardia; interventions that affect AV conduction may produce block but do not affect the tachycardia itself.
Digitalis toxicity often presents as atrial tachycardia with AV block and, while the mechanism is felt to be triggered automaticity, this arrhythmia is clinically indistinguishable from standard automatic atrial tachycardia. Thus, any unexplained atrial tachycardia with block should prompt a search for digitalis toxicity.
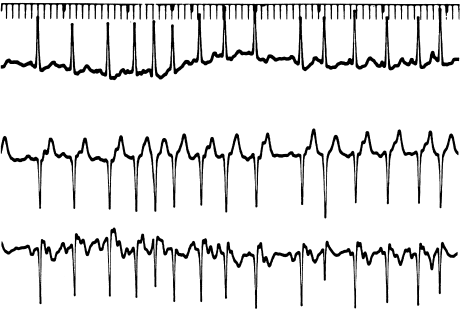
Multifocal (or chaotic) atrial tachycardia (Figure 6.1) is a form of automatic atrial tachycardia characterized by multiple (usually at least three) P wave morphologies and irregular PP intervals. It is thought to be due to multiple atrial automatic foci that are firing at differing rates. Multifocal atrial tachycardia is often seen with acute pulmonary disease and may be related to theophylline use.
Figure 6.1 Multifocal atrial tachycardia. This is an irregularly irregular rhythm with multiple (but discrete) P wave morphologies. It is thought to be due to multiple atrial automatic foci.
Inappropriate sinus tachycardia (IST) is a unique type of automatic tachycardia originating in the sinus node. Consequently, except for the clinical setting, IST is identical to normal sinus tachycardia (IST is discussed in detail in Chapter 11 and is mentioned here mainly to point out how it differs from other, more typical forms of automatic tachycardia). IST occurs in young, otherwise healthy patients who do not have reversible metabolic abnormalities, drug toxicities, or any other transient conditions producing the arrhythmia. Instead, IST is a chronic or persistent condition that is probably related to an underlying autonomic imbalance, and which can become quite debilitating over time.
For all forms of automatic supraventricular tachycardia, the basic principle of therapy is to attempt to reverse the underlying cause. Digitalis toxicity should be suspected in any patient who has been taking this drug, and digitalis should be withheld until toxic levels are ruled out. If the ventricular response is rapid enough to produce hemodynamic instability, the ventricular rate can usually be slowed with digitalis (if toxicity has been ruled out), verapamil, or β-blockers. In addition, if rapid 1 : 1 conduction is occurring during an automatic atrial tachycardia, an immediate slowing of the ventricular response can sometimes be accomplished by pacing the atria even faster than the rate of the tachycardia—fast enough to produce 2 : 1 or 3 : 1 AV block. Because these arrhythmias are not reentrant, they cannot be pace-terminated and usually do not respond well to direct current (DC) cardioversion. Class Ia antiarrhythmic drugs can be used in an attempt to terminate the arrhythmias, but, unless the underlying cause is reversed, these drugs tend to be relatively ineffective. The electrophysiology study offers no benefit in patients with automatic supraventricular tachyarrhythmias.
Reentrant Supraventricular Tachyarrhythmias
The vast majority of supraventricular tachyarrhythmias seen in ambulatory patients are due to reentry. In contrast to automatic atrial tachycardias, reentrant supraventricular tachycardias are seen in patients who are not acutely ill. Further, in contrast to reentrant ventricular tachyarrhythmias (see Chapter 7), reentrant supraventricular tachycardias are usually seen in patients who are free of chronic heart disease. Although reentrant circuits in the ventricle generally do not appear unless disease of the ventricular myocardium is present, the reentrant substrate for supraventricular arrhythmias tends to be congenital. Overt heart disease is therefore not necessary in order for a patient to develop these arrhythmias. Thus, the typical patient with reentrant supraventricular tachycardia is young and healthy.
There are five general categories of reentrant supraventricular tachyarrhythmia: AV nodal reentry, bypass tract-mediated macroreentry, intraatrial reentry, SA nodal reentry, and atrial flutter/atrial fibrillation. Many clinicians still tend to lump these reentrant arrhythmias (except for atrial flutter and atrial fibrillation) together into one large group called PAT (paroxysmal atrial tachycardia—generally, any regular, narrow-complex tachycardia with sudden onset and sudden termination). The term PAT, however, is a vestige from the days when the mechanisms for supraventricular arrhythmias were poorly understood. The electrophysiology study has now made it clear that PAT is almost always due to one of the categories of reentrant supraventricular tachyarrhythmia just listed. In many cases, the knowledgeable clinician can tell which type of supraventricular tachyarrhythmia he or she is dealing with (and therefore what type of therapy to use) merely by keeping these categories in mind while examining a 12-lead ECG of the arrhythmia.
AV Nodal Reentrant Tachycardia
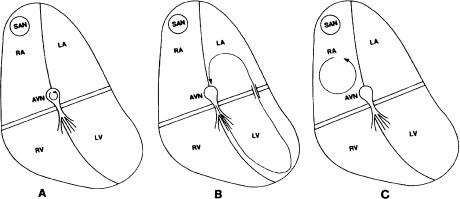
AV nodal reentrant tachycardia is the most common type of reentrant supraventricular tachycardia and is the operative mechanism in up to 60% of patients presenting with PAT. In AV nodal reentry, the reentrant circuit is usually said to be enclosed within the AV node (Figure 6.2a). In patients with AV nodal reentry, the AV node is functionally divided into two longitudinal pathways (dual AV nodal pathways). These dual pathways form the reentrant circuit. Because, for all practical purposes, the reentrant circuit in AV nodal reentry involves the AV node exclusively, this arrhythmia responds well to autonomic maneuvers and drugs that affect the AV node (digitalis, calcium blockers, and β-blockers).
Figure 6.2 The three most common reentrant supraventricular tachycardias. (a) AV nodal reentrant tachycardia. In this arrhythmia, the reentrant circuit is small and is thought to be localized entirely within the AV node. (b) Bypass tract-mediated macroreentrant tachycardia. An AV bypass tract serves as one pathway (usually the retrograde) and the normal AV conduction system serves as the other of this large (hence, macroreentrant) reentrant circuit. (c) Atrial reentrant tachycardia. In this arrhythmia, the reentrant circuit is contained within the atrial myocardium.
Bypass Tract-Mediated Macroreentrant Tachycardia
Bypass tract-mediated macroreentrant tachycardia is the next most common type of arrhythmia presenting as PAT (30%). In macroreentrant tachycardia, an AV bypass tract is present. (The majority of patients presenting with macroreentrant tachycardia do not have overt Wolff–Parkinson–White syndrome. Instead, they have concealed bypass tracts—bypass tracts that are incapable of conducting in the antegrade direction and therefore generate no delta waves.) In these patients, the bypass tract acts as one pathway (almost always the retrograde pathway), and the normal AV conducting system acts as the other (the antegrade pathway), of the reentrant circuit (Figure 6.2b). Because this reentrant circuit is a large circuit involving the AV node, the His–Purkinje system, and the ventricular and atrial myocardia as well as the bypass tract, it is termed a macroreentrant circuit. Also, because the circuit consists of several types of cardiac tissue, it can be attacked on many levels by many types of drug.
Intraatrial Reentry
Intraatrial reentry accounts for only a small percentage of patients presenting with PAT. In intraatrial reentry, the reentrant circuit is entirely within the atrial myocardium and does not involve the AV conducting system (Figure 6.2c). It resembles automatic atrial tachycardia in that discrete P waves, which usually differ from normal sinus P waves, precede each QRS complex, and AV block can occur without affecting the arrhythmia itself. It differs from automatic arrhythmias through its paroxysmal onset and offset and the fact that it can be induced and terminated by pacing. Class Ia drugs are often effective. Because the AV node is not part of the reentrant circuit, attacking the AV node does not affect this arrhythmia.
SA Nodal Reentry
SA nodal reentry is a fairly uncommon arrhythmia in which the reentrant circuit is thought to be enclosed within the SA node. Discrete P waves identical to sinus P waves precede each QRS complex. SA nodal reentrant tachycardia is distinguishable from both normal sinus tachycardia and IST by its onset, which is paroxysmal and does not display warm-up, by its equally sudden termination, and by the fact that it is inducible and terminable with pacing. Because the reentrant circuit is enclosed within the SA node, autonomic maneuvers and drugs such as digitalis, calcium blockers, and β-blockers tend to ameliorate this arrhythmia.
Atrial Flutter and Atrial Fibrillation
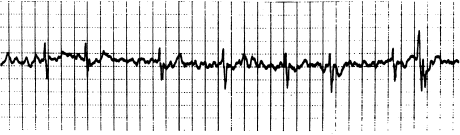
Finally, atrial flutter and atrial fibrillation are forms of reentrant atrial tachycardia. These arrhythmias are generally distinctive enough not to be considered to be PAT by clinicians who still use this terminology. In atrial flutter, the atrial rate is regular and in excess of 220 beats/min, and usually displays a typical sawtooth pattern (Figure 6.3). Atrial flutter can usually be terminated by pacing. Atrial flutter is virtually always accompanied by AV block, usually in a 2 : 1 pattern. In atrial fibrillation, the atrial activity is continuous and chaotic, and definite P waves cannot be distinguished (Figure 6.4). The ventricular response is irregular, reflecting the chaotic atrial activity. Both atrial flutter and atrial fibrillation are similar to intraatrial reentry (and to automatic atrial tachycardia) in that attacking the AV node does not directly affect these arrhythmias. In general, therapy is aimed at either converting the patient to normal sinus rhythm (with class I or class III drugs or cardioversion, or both) or simply controlling the ventricular response with AV nodal blocking agents.
Figure 6.3 Atrial flutter. One surface ECG lead and the right atrial electrogram are shown. Note the rapid, regular atrial rate (cycle length approximately 200 msec) and the 2 : 1 ventricular response.
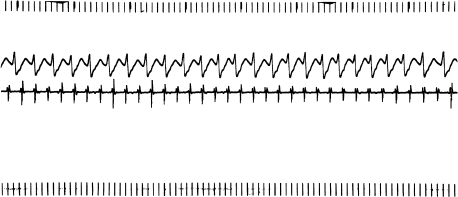
Figure 6.4 Atrial fibrillation. Note the chaotic atrial activity, absence of discrete P waves, and irregularly irregular ventricular response.
In summary, reentrant supraventricular tachyarrhythmias include several types of arrhythmia, which have different therapies, depending on the location and characteristics of the respective reentrant circuits. Most of these arrhythmias are commonly lumped together as PAT, a practice that is counterproductive to appropriate therapy. Once these arrhythmias are understood, the proper diagnosis can often be made (and proper therapy initiated) without resorting to invasive testing. The electrophysiology study, however, can be vital in managing patients with reentrant supraventricular tachyarrhythmias, especially because the majority of these arrhythmias can now be cured with transcatheter ablation techniques (see Chapter 8).
General Outline of The Electrophysiology Study In Reentrant Supraventricular Tachyarrhythmias
The key to studying supraventricular tachyarrhythmias in the electrophysiology laboratory is the capability of inducing these arrhythmias with programmed pacing techniques, while at the same time recording intracardiac electrograms from various key locations within the heart. As noted in Chapter 2, inducing any reentrant rhythm requires the existence of an appropriate anatomic substrate to form a reentrant circuit. In supraventricular tachyarrhythmias, the reentrant circuits can incorporate SA or AV nodal tissue, bypass tracts, and atrial and ventricular myocardial tissue. By recording strategically placed intracardiac electrograms and studying the patterns of activation, by examining the mode of initiation and termination of arrhythmias, and by studying the response to programmed pacing, most supraventricular tachyarrhythmias can be fully characterized in the electrophysiology laboratory. Specifically, the electrophysiology study seeks to determine the anatomic location and electrophysiologic characteristics of the reentrant circuit, the propensity of the circuit to sustain arrhythmias, the characteristics of those arrhythmias, and the response of the reentrant arrhythmias to various therapies.
Positioning The Catheters
To localize the reentrant circuits in supraventricular tachyarrhythmias, it is important to record electrograms from all four major cardiac chambers, as well as from the His-bundle region. These recordings can generally be obtained by positioning four electrode catheters: one in the right atrium, one in the right ventricle, one in the His position, and one in the coronary sinus (to record both left atrial and left ventricular deflections; Figure 6.5). With catheters in these four positions, one can record electrograms from the most critical locations and introduce paced impulses from the right ventricle and from both atria (pacing from the coronary sinus catheter generally produces capture in the left atrium alone).
Figure 6.5 Baseline recordings during a typical electrophysiology study for supraventricular tachycardia. Surface leads I, II, and V1 allow estimation of the QRS axis. The high right atrial (HRA) electrogram records activity near the SA node. The His-bundle electrogram is recorded from several electrode pairs on the His catheter (three in this example). Two recordings are made from the coronary sinus (both of which record left atrial and left ventricular activity)—coronary sinus distal (CSD) is recorded from the distal electrode pair on the coronary sinus catheter and coronary sinus proximal (CSP) is recorded from the proximal pair of electrodes. Finally, a recording is made from the right ventricular apex (RVA). With this combination of recordings, the electrophysiologist can keep track of events in all four cardiac chambers, as well as in the normal AV conducting system.
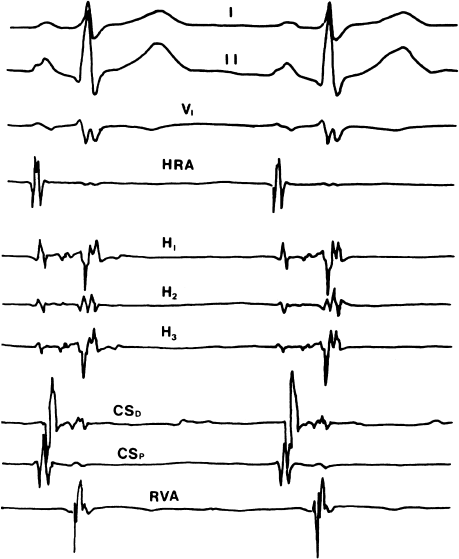
Evaluation of Supraventricular Tachyarrhythmias
Once the catheters are positioned, the characteristics of the arrhythmia can be deduced by answering four general questions. First, what is the mode of initiation and termination of the tachycardia? Second, what are the patterns of antegrade and retrograde activation during sinus rhythm and during tachycardia? Third, what is the evidence that atrial or ventricular myocardium is necessary to the reentrant circuit? Fourth, what are the effects of autonomic maneuvers and drugs on the tachycardia? These questions must be addressed for each type of supraventricular tachycardia that is induced during the study.
What Is The Mode of Initiation and Termination of The Tachycardia?
In assessing the mode of initiation of tachycardia, the electrophysiologist addresses several points.
First, is the tachycardia more readily inducible from one location than another? As noted in Chapter 4, inducing a reentrant arrhythmia requires the delivery of a premature impulse to the reentrant circuit at just the right moment. A major element influencing the inducibility of reentrant arrhythmias, then, is the distance between the pacing catheter and the reentrant circuit—the closer the catheter is to the circuit, the more likely it is that a paced premature beat will arrive at the circuit early enough to induce reentry. Thus, for instance, if a reentrant rhythm is significantly easier to induce with left atrial pacing (i.e. pacing from the coronary sinus) than it is with right atrial pacing, then the reentrant circuit may be expected to involve a left-sided bypass tract.
It is important to observe whether supraventricular tachycardia is readily inducible with ventricular pacing. Inducing tachycardia with ventricular pacing is readily accomplished in macroreentrant tachycardias, is relatively difficult in AV nodal reentry, and is rare with intraatrial reentry (in intraatrial reentry, the arrhythmia can be induced by ventricular stimulation only indirectly and infrequently, by means of retrograde stimulation of the atrium).
Another point relating to the mode of initiation of the tachycardia is the site of the conduction delay that typically occurs with the onset of reentrant supraventricular tachyarrhythmias. The reason for this conduction delay can be seen by reviewing the mechanism for inducing reentrant arrhythmias (see Figure 2.6). As described in Chapter 2, a reentrant circuit requires two roughly parallel pathways (labeled pathways A and B) connecting proximally and distally to common conducting tissues, forming an anatomic circuit. Pathway B has a longer refractory period than pathway A, and pathway A conducts impulses slower than pathway B. The initiation of reentry occurs when a premature impulse enters the circuit at a time when pathway B is still refractory from the previous normal impulse but pathway A has already recovered.
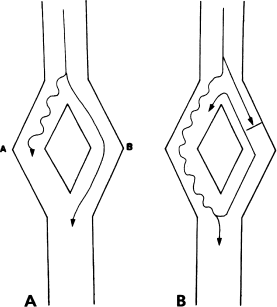
Note that, since pathway B conducts faster than pathway A, a normal (i.e. not premature) impulse will conduct via pathway B rather than pathway A (Figure 6.6a). When an early impulse initiates reentry, this impulse is blocked in pathway B (the fast pathway) and instead conducts via pathway A (the slow pathway). Thus, a beat that initiates reentry is commonly accompanied by conduction delay (Figure 6.6b). The electrophysiologist must pay particular attention to the occurrence of conduction delay in association with the onset of tachycardia. The site of this delay virtually always points to one of the pathways within the reentrant circuit.
Figure 6.6 Why reentry is accompanied by a conduction delay. (a) In this generic reentrant circuit, a normal impulse travels from top to bottom by the fastest available pathway (pathway B). No conduction delay is seen. (b) When a premature impulse blocks in pathway B and initiates reentry, that impulse must travel from top to bottom via the slow pathway (pathway A). Thus, an observer watching the exit of the impulse from the circuit will see a conduction delay whenever reentry is initiated.
An additional issue relating to the mode of initiation is the tachycardia (or echo) zone. The tachycardia zone is the range of coupling intervals of premature beats that will initiate reentrant tachycardia. As one introduces a series of premature impulses with progressively shorter coupling intervals, one impulse will eventually encounter the reentrant circuit during the effective refractory period (ERP) of pathway B (i.e. the impulse blocks in pathway B). At this point, reentry is often initiated. The latest premature impulse that blocks in pathway B (if it were any later, the impulse would not encounter refractory tissue) usually defines the beginning of the tachycardia zone. If one now continues to introduce premature impulses with progressively shorter coupling intervals, reentry will typically be induced with each succeeding premature beat until the ERP of pathway A is finally encountered. At this point, the premature impulse blocks in both pathways of the reentrant circuit and no further reentry occurs. The ERP of pathway A thus defines the end of the tachycardia zone. Note that the width of the tachycardia zone is related to the difference between the refractory periods of the two pathways constituting the reentrant circuit. The wider the tachycardia zone, the more likely a premature impulse will fall within that zone and induce reentry. During the electrophysiology study for supraventricular tachycardia, one thus tries to identify the refractory periods (and thus the tachycardia zone) of the various pathways involved in the reentrant circuit. By doing so, one can attempt to tailor therapy to reduce the width of the tachycardia zone.
The mode of termination of the arrhythmia is somewhat less helpful than the mode of initiation in localizing the bypass tract. Nonetheless, careful observation of the precise mechanism of termination of reentry can offer clues as to the type of reentrant rhythm. This point will be discussed in detail later.
What Are The Patterns of Antegrade And Retrograde Activation During Sinus Rhythm and During Supraventricular Tachycardia?
Abnormalities in the pattern of activation of impulses traveling from the atria to the ventricles and from the ventricles to the atria can help to localize portions of the reentrant circuit.
We have already discussed normal antegrade activation patterns (see Chapter 1). Impulses arising in the SA node traverse the atria in a radial fashion until they encounter the fibrous skeleton of the heart at the AV groove. There, the impulses are abolished, except in the AV conducting system (the AV node and His–Purkinje structures). Because of its electrophysiologic properties, impulses conduct relatively slowly through the AV node. Further, premature impulses conduct even more slowly (manifested in the His electrogram as a prolonged AH interval). Typically, if one introduces single atrial extrastimuli with progressively shorter coupling intervals, the delay in AV nodal conduction (and the AH interval) increases gradually. Eventually, the ERP of the AV node is reached and the early impulse is blocked.
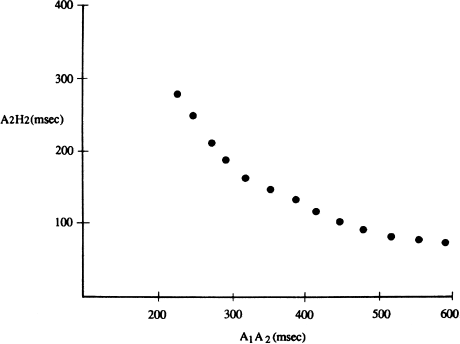
This normal pattern of gradually increasing AH intervals with progressively premature atrial impulses is shown graphically in Figure 6.7. This AV nodal conduction curve shows the normal smooth and continuous prolongation in AH intervals with progressively premature beats. This normal pattern can be disrupted by two general conditions that produce supraventricular tachycardias: dual AV nodal pathways and AV bypass tracts. These two conditions will be discussed in detail later.
Figure 6.7 A normal AV conduction curve. With progressively shorter coupling intervals, premature atrial beats display a progressive conduction delay in the AV node. In this graph, the conduction intervals (A1A2) are shown on the x axis, and the resulting AH intervals (A2H2) are shown on the y axis. The normal AV conduction curve is smooth and continuous, as shown.
When an impulse is conducted from the atria to the ventricles via the normal AV conducting system, ventricular activation follows a typical pattern. First, the intraventricular septum is depolarized, then the apices of the right and left ventricles, then the ventricular free walls, and finally the basilar portions of the ventricles. The area of the ventricles that is depolarized last is the left ventricular posterobasilar area. This normal pattern of ventricular activation is altered if there is a bypass tract that preexcites the ventricle. Studying the ventricular activation pattern in the electrophysiology laboratory can help to localize the ventricular insertion of a bypass tract.
The characteristics of normal retrograde conduction (i.e. the conduction of impulses from the ventricles to the atria that occurs during ventricular pacing) are important in evaluating supraventricular tachycardias. Normally, retrograde conduction occurs over the normal AV conducting system—the impulse travels from the ventricular myocardium to the Purkinje fibers, then to the His bundle, to the AV node, and finally to the atria, where it spreads in a radial fashion (i.e. from the atrial septum to the right and left atria). Although some retrograde conduction can be seen in most individuals, in most cases retrograde conduction is not as efficient as antegrade conduction. Thus, in most individuals, retrograde block occurs at longer coupling intervals than antegrade block (this is not always the case, however—e.g. a substantial minority of patients with complete AV block have intact retrograde conduction).
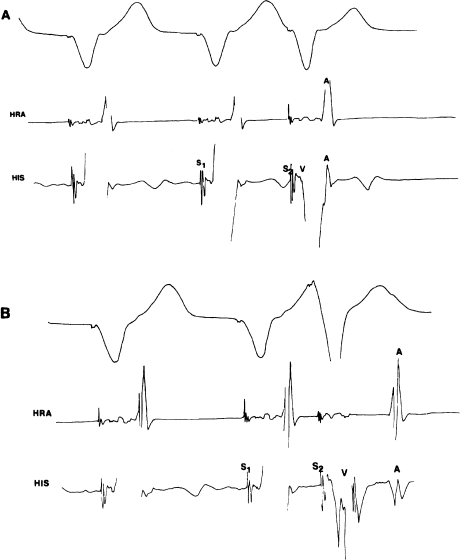
In normal individuals, if one introduces progressively earlier extrastimuli from the ventricle and measures the retrograde conduction intervals, one will see a progressive prolongation of the VA interval (Figure 6.8), similar to the gradual prolongation in the AH interval that one sees with antegrade conduction. Normally, block will occur first in the His–Purkinje system. In practical terms, however, one is only rarely able to discern the H spike in the His electrogram during retrograde conduction, as it is usually hidden in the large V complex.
Figure 6.8 Normal VA conduction during right ventricular pacing. A surface ECG lead, a high right atrial (HRA) electrogram, and a His bundle electrogram are shown. (a) With a relatively long S1–S2 coupling interval, retrograde conduction to the atrium is rapid (the VA interval following the S2 is short). Note that the retrograde atrial impulse is recorded in the His electrogram (near the AV node) before it reaches the high right atrium. This suggests retrograde conduction via the normal AV conducting tissues. (b) With a shorter coupling interval, retrograde conduction still occurs, but it is slower (the VA interval following the S2 is longer than in the previous figure). This retrograde conduction delay with shorter coupling intervals is normal when retrograde conduction occurs via the normal AV conducting tissues.
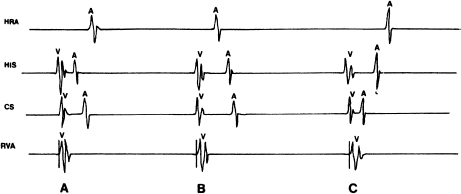
In studies of supraventricular tachyarrhythmias, the pattern of retrograde atrial activation is important. With normal retrograde conduction, the earliest atrial activation is seen in the His electrogram because that electrogram records the atrial activity near the AV node (i.e. the point of entry of the impulse into the atrial myocardium during normal retrograde conduction). The retrograde impulse then spreads radially across the atria, and the right atrial electrogram and left atrial electrogram are inscribed more or less at the same time (Figure 6.9a). This normal pattern of retrograde activation can be disrupted if an AV bypass tract is present. In this case, eccentric retrograde activation of the atria can occur. With a right-sided bypass tract, for instance, the right atrial electrogram will be inscribed earliest (Figure 6.9b); with a left-sided bypass tract, the left atrial electrogram will be inscribed earliest (Figure 6.9c). Mapping of retrograde atrial activation is one of the most useful methods for localizing AV bypass tracts.
Figure 6.9 Patterns of retrograde atrial activation are important for localizing AV bypass tracts. This figure shows electrograms from the high right atrium (HRA), the His bundle, the coronary sinus (CS), and the right ventricular apex (RVA) during pacing from the RVA. (a) The normal pattern of retrograde atrial activation. This pattern is seen when retrograde conduction occurs via the normal AV conducting system or with septal bypass tracts. The atrial septum is activated first (His electrogram), followed by left atrial (CS electrogram) and right atrial activation. (b) Retrograde activation pattern with a right-sided bypass tract. The right atrium is activated first because retrograde conduction occurs via a right-sided bypass tract. The septal atrium and the left atrium are then activated. (c) Retrograde activation pattern with a left-sided bypass tract. The left atrium is activated first (CS electrogram) because retrograde conduction occurs via a left-sided bypass tract. The atrial septum and the right atrium are then activated.
What Is The Evidence That Atrial or Ventricular Myocardium Is Necessary To The Reentrant Circuit?
Once a reentrant tachycardia has been induced, it can be helpful to attempt to deduce whether the atrial myocardium and ventricular myocardium are part of the reentrant circuit. One should always look for evidence of antegrade or retrograde block during the tachycardia. If either antegrade or retrograde block is seen to occur without affecting the cycle length of the tachycardia, macroreentry can be essentially ruled out.
For instance, in AV nodal reentrant tachycardia, retrograde stimulation of the atria occasionally occurs with a Wenckebach pattern (i.e. occasional retrograde beats are dropped). When this phenomenon is observed during a supraventricular tachycardia of constant cycle length, a reentrant circuit in which the atria are required for the maintenance of tachycardia can be ruled out.
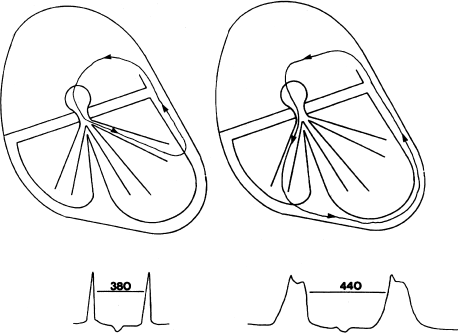
The effect of bundle branch block on the cycle length of the tachycardia can also be instructive. In AV nodal reentry, bundle branch block does not change the reentrant circuit because the circuit does not involve the bundle branches. In macroreentry, however, the sudden occurrence of bundle branch block on the same side as the AV bypass tract (such as left bundle branch block (LBBB) occurring during a macroreentrant tachycardia involving a left-sided bypass tract) will increase the cycle length of the tachycardia (Figure 6.10).
Figure 6.10 Effect of bundle branch block on macroreentrant tachycardia. In this figure, macroreentrant tachycardia involving a left-sided bypass tract is present. Initially, no bundle branch block is present, and the tachycardia cycle length is 380 msec. If LBBB occurs, it will now take longer for the reentrant impulse to reach the left-sided bypass tract, and the tachycardia cycle length will be prolonged to 440 msec.
What Are The Effects of Autonomic Maneuvers And Drugs on The Tachycardia?
When attempting to define the anatomic substrate of, and effective therapy for, reentrant supraventricular tachyarrhythmias, it is often helpful to take advantage of the differential effects of autonomic maneuvers and drugs on various cardiac structures. As noted in previous chapters, the AV node has rich autonomic innervation and is exquisitely sensitive to changes in both sympathetic and parasympathetic tone. In contrast, atrial and ventricular myocardial tissue responds moderately to changes in sympathetic tone but only minimally to changes in parasympathetic tone. Thus, vagal maneuvers, by increasing the refractory period and decreasing the conduction velocity of the AV node, can terminate reentrant arrhythmias in which the AV node is part of the reentrant circuit (such as AV nodal reentry and macroreentry). If the AV node is excluded from the reentrant circuit (as in intraatrial reentry), vagal maneuvers may increase heart block but will not directly alter the reentrant impulse.
Likewise, drugs that have a specific effect on the AV node (such as digitalis, calcium blockers, and β-blockers) can be effective in terminating or preventing reentrant arrhythmias that include the AV node as part of the circuit. In contrast, class Ia drugs have relatively little effect on the AV node but increase the refractory periods and slow conduction velocity in atrial and ventricular myocardium and in bypass tracts. Thus, reentrant circuits that involve these structures can be altered by class Ia drugs. Class Ib drugs affect the ventricular myocardium (and bypass tracts) but not atrial myocardium or the AV node. Because some supraventricular tachycardias (i.e. macroreentry) involve ventricular myocardium, the class Ib drugs may be useful in some cases. The class Ic drugs slow conduction in all cardiac tissues, including the AV node. Although offering relatively little help in identifying the anatomic substrate, class Ic drugs can be helpful in treating many forms of supraventricular tachyarrhythmia.
General Procedure For Performing The Electrophysiology Study In Patients With Supraventricular Tachyarrhythmias
When studying patients with supraventricular tachyarrhythmias, once the electrode catheters are in position most electrophysiologists begin with ventricular stimulation to study the characteristics of retrograde conduction. Although it may seem odd to begin with ventricular pacing when studying supraventricular arrhythmias, there are two reasons for doing so. First, many patients who have supraventricular tachycardias (especially those with bypass tracts) have a propensity for developing atrial fibrillation with atrial pacing. Atrial fibrillation, since it entails continuous and chaotic atrial activity, virtually precludes meaningful evaluation of other reentrant supraventricular arrhythmias. By first performing ventricular stimulation, the electrophysiologist hopes to avoid the early induction of atrial fibrillation. Second, for AV nodal reentry and for bypass tract-mediated macroreentry (the two most common forms of reentrant supraventricular tachyarrhythmia), retrograde conduction is a prominent and necessary component of reentry. Thus, the characteristics of retrograde conduction and the retrograde activation pattern of the atrium with ventricular stimulation are important in characterizing these arrhythmias. It is therefore logical to evaluate these characteristics as early as possible.
Generally, retrograde conduction is evaluated using the extrastimulus and incremental pacing techniques. Single ventricular extrastimuli are introduced (either in sinus rhythm or following a train of paced beats) with gradually decreasing coupling intervals until the extrastimulus fails to capture the ventricle. With each extrastimulus, the electrophysiologist notes the presence or absence of retrograde stimulation of the atrium, the pattern of atrial activation, and the conduction time of the retrograde impulse (the VA interval; see Figure 6.8). The coupling interval at which retrograde conduction is blocked (the retrograde ERP) is also noted. Next, incremental trains are introduced with progressively shorter cycle lengths. Note is taken of the cycle length at which 1 : 1 retrograde conduction no longer occurs.
After ventricular stimulation is completed, pacing is performed from the high right atrium. The procedure here is similar to that described in Chapter 5—atrial extrastimuli and incremental atrial pacing are used to assess the characteristics of AV conduction and of SA nodal function.
Next, similar stimulation is performed using left atrial pacing (i.e. with the coronary sinus catheter). This is done to look for evidence of a left-sided AV bypass tract.
When a bypass tract is suspected, pacing from multiple atrial sites is often performed (by moving the right atrial and/or coronary sinus catheters to various positions) to try to localize the bypass tract—the closer to the bypass tract one paces, the more preexcitation will occur.
For the vast majority of patients with reentrant supraventricular tachyarrhythmias, the reentrant arrhythmia will be induced during one or more of these pacing maneuvers. Whenever reentrant tachycardia is induced, the protocol is interrupted so that the characteristics of the arrhythmia can be studied. Specifically, the mechanism of the tachycardia is noted, the cycle length is recorded, an assessment of the patient’s hemodynamic stability and level of symptoms is made, and atrial or ventricular stimulation is carried out to examine the effect of such stimulation on the arrhythmia.
If reentrant supraventricular tachycardia is not induced with standard pacing techniques, multiple extrastimuli are used and drug administration (such as an isoproterenol infusion) is implemented to try to bring out the arrhythmia.
If a bypass tract is known or suspected to occur, the final step during the baseline (drug-free) study is to induce atrial fibrillation. This is done to assess the ability of the bypass tract to conduct rapidly enough during atrial fibrillation to pose a lethal risk to the patient.
Finally, at the end of the baseline study, after the reentrant circuits have been localized and the mechanism of tachycardia has been deduced, drug studies may be carried out in an attempt to devise effective therapy for the patient. Drug studies for supraventricular tachycardias, however, are becoming ever more rare now that most of these arrhythmias are routinely treated with ablation procedures.
Although this general outline of the electrophysiology study for supraventricular tachyarrhythmias is fairly representative, no two electrophysiologists follow precisely the same protocol. In fact, no single electrophysiologist follows precisely the same protocol in every patient. Even within the same general category of arrhythmias, supraventricular arrhythmias show tremendous variability from patient to patient. Thus, every electrophysiology study has to be individualized. Electrophysiologists usually approach these patients with a general outline of a protocol, tailoring the study as they go, depending on the findings.
With this introduction, we will now review the electrophysiologic evaluation of specific types of supraventricular tachyarrhythmia.
The Electrophysiology Study in AV Nodal Reentrant Tachycardia
As we have noted, AV nodal reentrant tachycardia is the most common type of arrhythmia presenting as PAT, probably accounting for 60% of all such arrhythmias. AV nodal reentry is seen in all age groups and occurs in both sexes equally. The incidence of underlying heart disease in patients with this arrhythmia is no greater than in the normal population.
Mechanism of AV Nodal Reentry
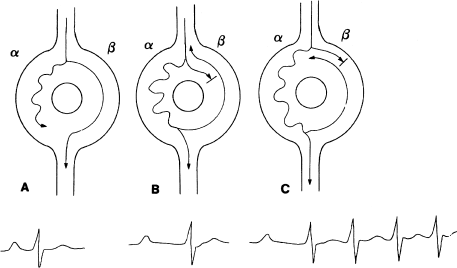
One can think of the AV node in patients with AV nodal reentrant tachycardia as being shaped like a doughnut (Figure 6.11). The AV node in these patients behaves functionally as if there were two separate pathways through the node. The two pathways (the alpha and the beta pathways) are differentiated by their characteristic electrophysiologic properties. The alpha pathway (or the slow pathway) usually has a relatively short ERP and conducts slowly. The beta pathway (or the fast pathway) usually has a relatively long ERP and conducts more rapidly. Patients whose AV nodes are arranged in such a fashion are said to have dual AV nodal pathways (see Chapter 8 for a more detailed discussion of the anatomy and physiology of dual AV nodal pathways).
Figure 6.11 AV nodal reentrant tachycardia. (a) In AV nodal reentry, the AV node is functionally divided into two pathways (alpha and beta). The alpha pathway conducts more slowly than the beta, and the beta pathway has a longer refractory period than the alpha. A normal atrial impulse reaches the ventricles via the beta pathway. (b) A premature atrial impulse can find the beta pathway refractory at a time when the alpha pathway is not. In this case, the premature impulse conducts via the alpha pathway. Because conduction down the alpha pathway is slow, the resultant PR interval is prolonged. (c) If conditions are right, a premature impulse can block in the beta pathway and travel down the alpha pathway (as in (b)), then travel retrograde up the beta pathway and reenter the alpha pathway in the antegrade direction. A circuitous impulse is thus established within the AV node, and AV nodal reentrant tachycardia results. Note the prolonged PR interval in the beat that starts AV nodal reentry (caused by jumping from the beta to the alpha pathway). This conduction delay is typically seen in reentrant supraventricular arrhythmias.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


