Evaluation of SA Nodal Abnormalities
Anatomy of the SA Node
The SA node can be thought of as a subendocardial, comma-shaped structure, approximately 3 mm in width and 10 mm in length, the head of which is located laterally to the right atrial appendage, near the junction of the atrium and the superior vena cava, on the crista terminalis. (The crista terminalis is an endocardial ridge extending like a narrow mountain chain from the superior to the inferior vena cava along the lateral right atrium.) The tail of the SA nodal “comma” extends downward along the crista terminalis, toward the inferior vena cava.
The SA node receives rich innervation from both sympathetic and parasympathetic fibers; accordingly, its function is strongly influenced by the autonomic nervous system. There is some evidence that autonomic tone helps to determine which portion of the SA node (i.e. the head or the tail) is “firing” at a given time—elevated sympathetic tone tends to trigger the head of the SA node, while elevated parasympathetic tone tends to trigger the tail.
SA Nodal Dysfunction
Disease of the SA node is the most common cause of bradycardia. The bradyarrhythmias that accompany SA nodal disease can manifest as intermittent or sustained sinus bradycardia, as sudden episodes of SA nodal arrest or exit block, or as sinus bradycardia alternating with paroxysms of atrial tachyarrhythmias (a condition referred to as “brady-tachy syndrome”). When sinus bradyarrhythmias are sufficient to produce symptoms, sick sinus syndrome is said to be present.
(Note that the “official” definition of sinus bradycardia can be misleading. While most textbooks define normal sinus rhythm as having a rate between 60 and 100 beats/min, this range is probably incorrect. Normal, healthy individuals often have resting sinus rates as low as 40; while resting heart rates in the upper 80s or 90s often indicate the presence of occult medical problems, such as anemia or cardiopulmonary or thyroid disorders.)
Clinical studies show that the bradycardia caused by SA nodal disease is usually benign. That is, people do not commonly die from sinus bradycardia. On the other hand, the presence of SA nodal disease is associated with increased mortality—but that excess mortality is often due to noncardiac causes.
Because sinus bradycardia is usually not lethal, the level of aggressiveness that ought to be used in evaluating and treating suspected SA nodal disease is solely dependent on whether any associated symptoms are thought to be present. If SA nodal disease is causing symptoms (i.e. if sick sinus syndrome is present), permanent pacing is indicated. If there are no symptoms, in general there is no indication for pacing.
By far the most common cause of SA nodal disease is simple fibrosis of the SA node; a condition that is associated with aging and often is also accompanied by diffuse fibrosis within the atria and the AV conducting system. Accordingly, most patients with SA nodal dysfunction are elderly. Other causes of SA nodal dysfunction include atherosclerotic disease involving the SA nodal artery; cardiac trauma, especially during surgical correction of congenital heart disease; cardiac infiltrative or inflammatory disorders; and thyroid disorders.
Brady-tachy syndrome deserves a special mention. This syndrome occurs because the diffuse atrial fibrosis that often accompanies SA nodal dysfunction can produce a propensity for atrial fibrillation or flutter. Individuals with brady-tachy syndrome thus will have intermittent episodes of atrial tachyarrhythmias, with intervening periods of sinus bradycardia. Importantly, because their diseased SA nodes often display exaggerated overdrive suppression (see Chapter 4 and later in this chapter), these patients tend to have very prolonged asystolic pauses when their tachyarrhythmias abruptly terminate. Quite often then, their presenting symptoms have little to do directly with either the tachycardia or the sinus bradycardia—instead they are frequently caused by this post-tachycardia asystole. Thus, patients with brady-tachy syndrome can have sudden and relatively severe episodes of lightheadedness, or even frank syncope. Furthermore, because the atrial fibrosis also commonly involves the AV conduction system, during their atrial tachyarrhythmias these patients may have surprisingly well-controlled (or even slow) ventricular responses. Seeing an unexpectedly slow ventricular response in a patient with atrial fibrillation should thus immediately raise the question of a generalized disorder of the conducting system—and the physician should be prepared to administer immediate pacing therapy if cardioversion is contemplated.
Patients with brady-tachy syndrome often end up with chronic atrial fibrillation accompanied by a reasonably well-controlled ventricular response. Indeed, one might be tempted to view this chronic atrial fibrillation as “God’s way” of guaranteeing an adequate heart rate in the face of diffuse conducting system disease. While this may be so, in the intervening years patients are often very symptomatic, not to mention at risk for syncope-induced trauma, so sitting on one’s hands and waiting for this final, relatively stable rhythm to occur does not constitute adequate therapy.
Although the electrophysiology study can help to document the presence of SA nodal dysfunction, it generally cannot help to assess whether such dysfunction is causing symptoms—or, therefore, whether pacing therapy is indicated. One is, however, occasionally faced with a patient who has symptoms suggesting significant bradycardia (such as lightheadedness or syncope), without clearly documented bradycardia. In such circumstances, the electrophysiology study can be helpful in determining whether or not the SA node is intrinsically normal.
Evaluating SA Nodal Function in the Electrophysiology Laboratory
Sinus Node Recovery Time (SNRT)
A primary manifestation of SA nodal dysfunction is disordered automaticity, where the slope of phase 4 automaticity is reduced in the SA node, resulting in bradycardia.
The test designed to assess SA nodal automaticity in the electrophysiology laboratory is the “sinus node recovery time” (SNRT; Figures 5.1 and 5.2). Measurement of the SNRT is based on the phenomenon of overdrive suppression. Overdrive suppression is the temporary slowing of automaticity seen when an automatic focus is exposed to rapid, extrinsic electrical stimuli. When this overdriving stimulation stops, it takes the automatic focus a few cycles to recover its normal rate of discharge. Until it recovers, it fires more slowly than its original baseline rate; thus, the automatic focus has been transiently “suppressed” by overdrive stimulation. Overdrive suppression is a normal behavior of automatic foci; in SA nodal disease, however, overdrive suppression tends to be exaggerated.
Figure 5.1 A normal sinus node recovery time (SNRT). This figure shows three surface ECG leads (top three tracings), a right atrial (RA) electrogram (fourth tracing), and a His-bundle electrogram (fifth tracing). In measuring the SNRT, only the RA electrogram needs to be examined. The first three impulses on the RA electrogram represent the final three paced beats during 30 seconds of incremental pacing. Pacing is stopped, and the interval from the last paced atrial complex to the first spontaneous atrial complex on the RA electrogram is measured. In this instance, the basic cycle length is 800 msec and the SNRT equals 1260 msec. This is a normal value.
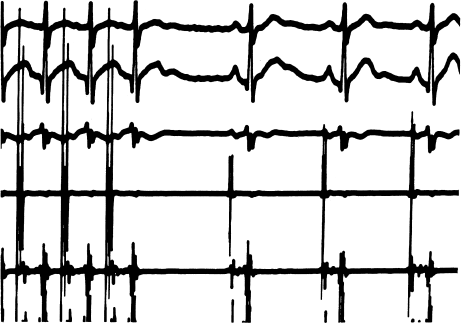
Figure 5.2 An abnormal sinus node recovery time (SNRT). This figure shows three surface ECG leads and the right atrial (RA) electrogram. The last three paced impulses are shown. The interval following termination of pacing (from the RA electrogram) is 2200 msec, an abnormally long SNRT. Note that during the recovery interval a premature ventricular complex (PVC) is seen. Because this PVC does not conduct retrogradely (this is obvious because no corresponding atrial activity is present on the RA electrogram), the PVC does not affect the sinus node and therefore does not affect measurement of the SNRT.
In nature, as we have seen, overdrive suppression of the SA node is caused by atrial tachyarrhythmias. In the electrophysiology laboratory, we provoke it with a temporary pacemaker.
Measuring SNRT
Measuring the SNRT is simple in concept and in practice, although interpreting the results can be more challenging. An electrode catheter is placed in the high right atrium near the SA node and pacing is initiated to overdrive the SA node. After a while, pacing is terminated and the ensuing “recovery” time from the last paced beat to the next spontaneous sinus beat is measured.
In the formal measurement of SNRT, several pacing sequences are tested, beginning with a pacing rate just slightly faster than the basic sinus rate, and generally ending with a cycle length of 300 msec (200 beats/min.) Each pacing sequence is maintained for at least 30 seconds to maximize overdrive suppression—and is then abruptly stopped. The first recovery interval (the interval from the last paced atrial complex to the first spontaneous SA nodal depolarization) is normally longer than the pre-pacing baseline sinus interval, reflecting the degree of overdrive suppression induced by pacing. After pacing is stopped, the SA node gradually returns to its baseline rate over five to six beats. In SA nodal disease, two observations are commonly seen. First, the initial recovery interval can be longer than normal. Second, the gradual return to the baseline sinus rate can be interrupted during subsequent recovery intervals by marked “secondary pauses.”
By the end of the study, several initial recovery intervals will have been measured: one at each pacing rate. In addition, one or more secondary pauses may have been observed. The official SNRT is deemed to be the longest recovery interval observed during the entire test, whether it is one of the initial recovery intervals or a secondary pause.
One might think that the longest post-pacing recovery times would always be seen after the more rapid pacing rates, but this is often not the case. Recovery times measured after slower pacing rates are frequently longer than those measured after faster pacing rates. This phenomenon—longer recovery times after slower overdrive suppression—is attributed to the conduction properties of the SA node itself. If conduction within the perinodal cells (cells that surround the pacemaker cells) is abnormal then SA nodal “entrance block” can occur, wherein some of the paced impulses are blocked from reaching the pacemaker cells. Faster pacing rates can thus actually result in fewer impulses reaching the SA nodal pacemaker cells themselves, and hence in less-effective overdrive suppression, than slower pacing rates. This is why a wide range of pacing rates, instead of just the more rapid pacing rates, are used when measuring SNRT. (Assessment of the conduction properties of the SA node is discussed later in this chapter.)
In any case, determining the SNRT is easy enough; deciding whether that SNRT is normal or abnormal is a little more problematic. One difficulty is that overdrive suppression of the SA node, being a normal phenomenon, occurs in everybody. Unfortunately, there is wide variation in SNRTs among apparently normal individuals as well as considerable overlap in measured SNRTs between patients with apparently normal and clearly abnormal SA nodes.
Deciding on the upper normal values for the SNRT, then, is not dictated by nature, but by electrophysiologists. “Normal” values vary from laboratory to laboratory, depending on whether electrophysiologists want their measurements to err on the side of sensitivity (in which case a lower value would be used) or specificity (in which case a higher value would be used). A conservative upper limit of “normal” for the SNRT, above which most experts would agree that SA nodal dysfunction is present, is 1500 msec.
Another difficulty in interpreting SNRTs is the fact that the SNRT is related to the underlying heart rate (or the basic cycle length, BCL), such that at slower underlying heart rates, a longer recovery time is normally seen. An SNRT that is abnormal at a shorter BCL could be normal at a longer BCL.
To account for this relationship between SNRT and BCL, electrophysiologists have created two indices, one or both of which are now measured routinely during electrophysiology studies. The first is the corrected sinus node recovery time (CSNRT), which is calculated simply by subtracting the patient’s BCL from the SNRT. Thus:
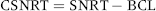
By convention, the upper limit of “normal” for the CSNRT is 525 msec. In other words, the SNRT should be no more than 525 msec longer than the BCL.
The second index commonly used is the ratio of the SNRT to the BCL (SNRT/BCL × 100%). A ratio greater than 160% is usually considered abnormal.
Figure 5.1 demonstrates a normal SNRT. Here, the patient’s BCL is 800 msec and the initial recovery interval is 1260 msec. Thus, the SNRT is 1260 msec, the CSNRT (SNRT − BCL) is 460 msec, and the ratio of the SNRT to the BCL is 158%. These values are all within normal limits.
An abnormal SNRT is demonstrated in Figure 5.2. The BCL in this example is also 800 msec. The longest recovery interval (and thus the SNRT) is 2200 msec. The CSNRT is 1400 msec, and the ratio of the SNRT to the BCL is 275%. All of these values are abnormal.
These two examples are straightforward in that all the SNRT measures—the SNRT itself, the CSNRT, and the ratio—are either normal (Figure 5.1) or abnormal (Figure 5.2). Not uncommonly, there will be divergence among these three measures. In such cases, the electrophysiologist must use his or her clinical judgment in interpreting the test. Given the relatively arbitrary nature of determining normal from abnormal SNRT values in the first place, this circumstance should not add appreciably to one’s level of discomfort. Indeed, once you resort to the electrophysiology study for determining whether a patient’s SA node is normal or not, you are signing up for a certain amount of arbitrariness.
Sinoatrial Conduction Time (SACT)
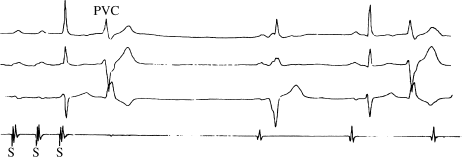
The sinoatrial conduction time (SACT) test is meant to assess how well the SA node is able to conduct the electrical impulses it produces out to surrounding atrial tissue. The idea that the SA node might not always allow generated impulses to reach the atria came from the observation of SA nodal exit block (see Figure 5.3). In exit block, a sudden sinus pause occurs with a duration that is exactly twice the normal sinus cycle length, suggesting that an impulse was indeed generated at the right time, but that it failed to conduct out of the SA node. Occasional episodes of exit block are relatively common in patients with SA nodal dysfunction. (SA nodal exit block is analogous to the entrance block we discussed in the section on SNRT, and reflects the same physiology.)
Figure 5.3 This rhythm strip illustrates SA nodal exit block. Note the sudden sinus pause, with a duration that is almost exactly twice the normal sinus cycle length. Presumably, the SA nodal pacemaker cells fired at the appropriate time (indicated by the asterisk), but the impulse was blocked from exiting the SA node thanks to abnormal perinodal conduction. Patients with SA nodal dysfunction commonly display occasional episodes of exit block. These patients usually also have abnormal SACT measurements (see text).

To understand how such an exit block might occur and how the SACT is measured, it is helpful to visualize the SA node as a small patch of pacemaker cells surrounded by a rim of specialized perinodal tissue that has electrophysiologic characteristics similar to those of the AV node (Figure 5.4). To exit from the SA node, a generated impulse must pass through this rim of perinodal tissue; if conduction is abnormal, exit block may occur.
Figure 5.4 Measurement of sinoatrial conduction time (SACT). (a) The SA node consists of a patch of pacemaker cells surrounded by a rim of perinodal tissue, which conducts electrical impulses slowly (similarly to the AV node). To measure SACT, an electrode catheter is positioned near the SA node and a premature impulse is delivered. The SACT can be deduced by measuring the interval from the premature paced impulse to the next spontaneous impulse (i.e. the return cycle). This return cycle consists of the conduction time into the SA node (b), the normal SA nodal depolarization time (i.e. the basic cycle length, BCL) (c), and the conduction time out of the SA node (d). (e) illustrates the resultant right atrial electrogram. The SACT is half the difference between the return cycle and the BCL.
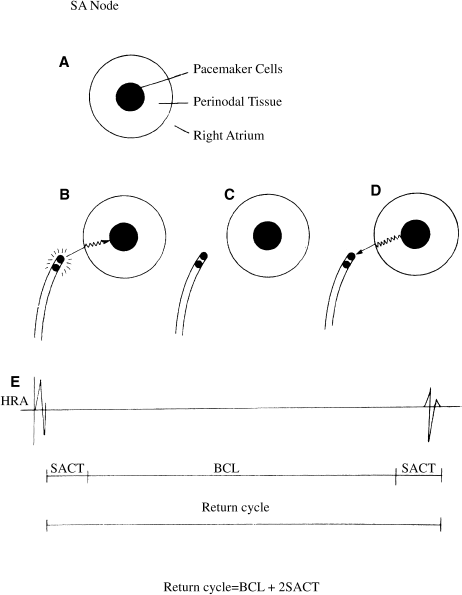
The SACT is a measure of the time it takes an impulse originating in the SA node to travel through the perinodal tissue and into the atria. Prolonged SACTs are thought to indicate abnormal conduction through this tissue, and thus a propensity for SA nodal exit block.
To measure SACT, an electrode catheter is placed in the high right atrium near the SA node and atrial pacing is performed to depolarize and thus reset the SA node. The subsequent return interval (i.e. the time from the last paced atrial depolarization to the first spontaneous SA nodal depolarization, as measured from the high right atrial electrode catheter) is assumed to reflect the sum of the time it takes for the paced impulse to penetrate into the SA node (at which time it resets the SA node) plus the basic sinus cycle length plus the time it takes for the subsequent spontaneous beat to penetrate out of the SA node. Assuming that the times of penetration into and out of the SA node are equivalent:
Because the BCL is known and the return interval can be measured, the SACT can be calculated.
In Figure 5.5, the basic cycle length is 800 msec. The paced beat (A2) is assumed to penetrate and reset the SA node, and the return interval is 900 msec. From this formula, 900 msec = 800 msec + 2 SACT. The SACT is thus 50 msec. A normal SACT is considered to be approximately 50–125 msec.
Figure 5.5 Measurement of sinoatrial conduction time (SACT, Strauss method). One surface ECG lead and a right artial (RA) electrogram are shown. Measurements are made from the RA electrogram. A single premature atrial impulse is delivered (A2) at a coupling interval of 780 msec: slightly faster than the basic cycle length (BCL) of 800 msec. The return interval (A2–A3) is 900 msec, which is the BCL + 2 SACT. The SACT is thus 50 msec.
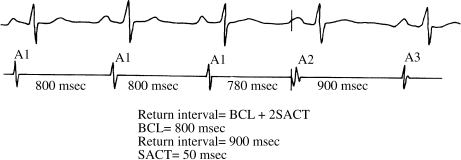
Two methods have been developed for measuring SACT. In the Narula method, short trains of slow atrial pacing are used, barely faster than the basic sinus rate. A rate just faster than the basic sinus rate is used to minimize any overdrive suppression. (Inducing overdrive suppression would invalidate the SACT calculation, since the SA node would no longer be discharging at its BCL.) In the Strauss method, a series of single premature atrial impulses are used, to guarantee that no overdrive suppression will occur. The principles of both methods, however, are as described earlier. Whichever method is used to measure SACT, the main limitation of this test is that many patients with SA nodal dysfunction have modestly irregular BCLs, which can introduce considerable error into the SACT calculation.
Intrinsic Heart Rate (IHR)
As previously mentioned, the SA node is richly innervated by both sympathetic and parasympathetic fibers. Occasionally, it can therefore be difficult to determine whether suspected SA nodal dysfunction is actually intrinsic to the SA node, or whether it might be due to abnormal autonomic tone. In such cases it can be helpful to measure the intrinsic heart rate (IHR).
The IHR is assessed by administering drugs to block both branches of the autonomic nervous system then measuring the resultant heart rate. Classically, autonomic blockade is accomplished by giving both propranolol (0.2 mg/kg) and atropine (0.04 mg/kg). According to the formula devised by Jose, after autonomic blockade the normal IHR = 118.1 − (0.57 × age).
Patients presenting with sinus bradycardia but who turn out to have normal IHRs are considered to have normal intrinsic SA nodal function; their bradycardia is apparently due to autonomic influences. Patients with bradycardia and depressed IHRs are considered to have intrinsic SA nodal dysfunction. Alternately, patients with inappropriate sinus tachycardia (see Chapter 11) often have substantially elevated IHRs.
In clinical practice, it is rarely necessary to measure IHR in order to document intrinsic SA nodal disease. An exercise test, for instance, is a much simpler way to accomplish parasympathetic withdrawal in a patient presenting with sinus bradycardia, and a blunted heart-rate response to exercise (in the absence of drugs that cause bradycardia, such as β-blockers and calcium blockers) usually clinches the diagnosis of intrinsic SA nodal dysfunction.
Interpreting SA Nodal Tests: When to Carry Out Electrophysiologic Testing
When assessing patients for SA nodal dysfunction, several simple points bear keeping in mind. First, SA nodal dysfunction is generally not a lethal condition, and therapy is necessary only to the extent that it produces symptoms. Second, the best way to diagnose SA nodal dysfunction is to see it occurring spontaneously, because the specificity of such an observation is 100%. Thus, ambulatory cardiac monitoring (and not electrophysiologic testing) is the study of choice in assessing SA nodal dysfunction. Third, since the electrophysiology study can help to determine only whether SA nodal dysfunction is present (and not whether it causes symptoms), once unexplained sinus bradyarrhythmias are already known to occur, there is generally no reason to consider electrophysiologic testing. Fourth, while the electrophysiology study offers methods for evaluating both the automaticity (SNRT) and conductive properties (SACT) of the SA node, the specificities and sensitivities of these tests as usually applied are estimated to be only approximately 70%.
With these points in mind, we can confidently state the following guidelines in assessing SAnodal disease:
Evaluation of AV Conduction Disorders
AV conduction disorders are the second major cause of bradyarrhythmias. As with SA nodal disease, the clinician’s chief concern with AV conduction disease is deciding whether the affected patient should receive a pacemaker. This decision is based on three essential pieces of data: whether the conduction disorder is producing symptoms; the site of the conduction disorder; and the degree of conduction block. Once again, thanks to what has been learned in the electrophysiology laboratory, we usually do not need to perform invasive testing to make this decision.
Symptoms of AV Conduction Disorders
The symptoms that occur with AV conduction disease are the same as those that occur with any bradyarrhythmia: lightheadedness, dizziness, presyncope, and syncope. Whatever the site or degree of block, AV block that produces any of these symptoms needs to be treated. Often, however, especially with first- or second-degree block, AV conduction disturbances are totally asymptomatic.
The Site of the Conduction Disorder
Determining the site of the conduction disturbance is important for a simple reason: block that occurs in the AV node (proximal block) is usually benign, whereas block that occurs in the His–Purkinje system (distal block) is potentially lethal.
AV Nodal Block
Conduction disturbances in the AV node most often have acute, transient, and reversible causes. Ischemia or infarction involving the right coronary artery (which gives off the AV nodal artery in 90% of patients) can cause AV nodal block. Thus, heart block following an inferior myocardial infarction is usually localized to the AV node, and normal AV conduction almost always recovers (though block can persist for days or even weeks). Acute rheumatic fever and other cardiac inflammatory conditions can also produce transient AV nodal block. While drugs that affect AV nodal function—mainly digoxin, β-blockers and calcium blockers—may produce first-degree AV block, higher degrees of drug-induced AV nodal block suggest underlying intrinsic AV nodal dysfunction.
Complete heart block localized to the AV node is usually accompanied by the emergence of a relatively reliable escape pacemaker, located just distally to the AV node. This “high junctional” escape rhythm often yields a baseline heart rate of 40–55 beats/min, and responds at least moderately well to increases in sympathetic tone. Congenital complete heart block, which is usually localized to the AV node, illustrates the typical, relatively benign character of AV nodal block.
Since AV nodal block is usually reversible, it can frequently be managed simply by dealing with the underlying cause, although sometimes temporary pacing support is required in the meantime.
His–Purkinje Block
In stark contrast to AV nodal block, block occurring distally to the AV node is potentially life-threatening. The potential lethal effect of distal heart block can largely be attributed to the unreliable, unstable, and slow escape pacemakers that tend to accompany this condition. These distal escape pacemakers usually discharge irregularly, 20 to 40 times per minute, and are prone to fail altogether. Thus, syncope, hemodynamic collapse, and death are much more likely to occur when AV block is located in the His–Purkinje system. Furthermore, distal AV block tends to be chronic and progressive in nature, instead of transient and reversible.
Distal AV block following myocardial infarction is almost always associated with occlusion of the left anterior descending artery, and thus with anterior myocardial infarctions. His–Purkinje block can also be seen with inflammatory and infiltrative cardiac disease, with myocardial fibrosis, and in association with aortic valve and mitral valve calcification.
The Degree of AV Block
First-Degree AV Block
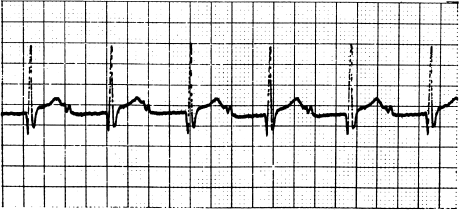
In first-degree AV block, all atrial impulses are transmitted to the ventricles, but with prolonged conduction times (Figure 5.6). First-degree block is diagnosed from the ECG; all P waves are conducted, but the PR interval is prolonged (usually to between 0.20 and 0.40 seconds, but sometimes much longer.) In most patients with first-degree AV block, slow conduction is localized to the AV node; slow conduction in the His–Purkinje system can, however, occasionally cause first-degree block. First-degree AV block usually causes no symptoms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree