Fig. 4.1
Cost of medical therapy for end-stage heart failure patients during last 2 years of life by 3 month time periods as a percentage of the total cost
4.2.2 Surgical Therapy
The cost of surgical therapy is also expensive but associated with trends showing a reduction in cost with increased experience. Heart transplantation is a standard form of surgical treatment of end-stage heart failure. In Japan only 169 patients were placed on the 2010 heart transplant list. In 2009, only four heart transplants were performed and only 69 between 1998 and June 2010. Two-thirds of patients on the transplant list have been waiting more than 2 years for a heart transplant with nearly 15 % waiting more than 5 years [3].
At Columbia University Medical Center, the overall average cost of heart transplant with 120 days follow-up was reported as $150,000. Despite its expense, the societal cost of heart transplant will never be prohibitively high simply because the volume of transplantations is primarily determined by the limited availability of donor hearts [12].
Increasing experience with this procedure and advances in immunosuppression were associated with 45 % reduction in cost reported for heart transplantation of nearly 45 % between 1991 and 1995, shortly after Center for Medicare/Medicaid Services (CMS) began to approve heart transplantation. Similarly a 40 % reduction in mechanical support cost was reported over a 3 year experience period between 2001 and 2004 (Fig. 4.2) after this therapy received CMS approval [13].
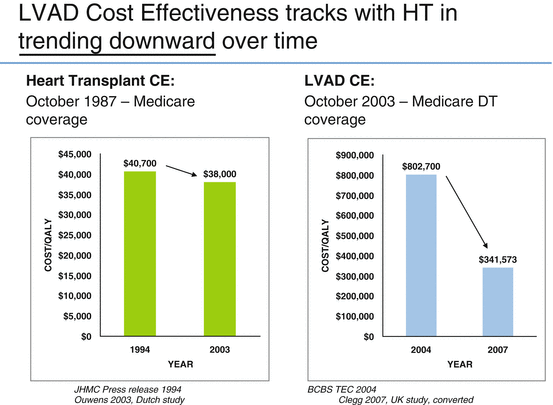

Fig. 4.2
Reduction in cost of heart transplantation over 4 year time period 1991–1995 versus reduction in cost of LVAD therapy from 1991 to 1994
Use of mechanical circulatory assist devices have become an increasing surgical treatment option for patients who deteriorate while awaiting heart transplant as well as an alternative to transplant. The cost of the index hospitalization for the patients who were randomized to LVAD therapy in the REMATCH study averaged $210,000, with a large standard deviation of $193,000 and a median cost of $145,000 (range $76,000–$733,000). The cost was approximately $100,000 for those who survived the index hospitalization and as high as $700,000 for those who had an extended intensive care unit period and did not survive hospitalization, which emphasizes the importance of patient selection in use of this therapy [14].
A more recent comparison of the cost of mechanical support for patients receiving a LVAD between 2003 and 2004 compared to patients implanted in the year 2000 showed that the length of stay had been reduced by over 25%, and the index hospitalization costs were reduced by approximately 40 % to an average of $128,000 [13]. This was in part due to a 40 % reduction in length of stay and a 44 % reduction in hospital cost; this averaged $115,000 in survivors. Slaughter et al. also reported a similar 45 % reduction in cost of the index hospitalization over a 2-year period of observation, which averaged $125,000 in their recent assessment [15].
The use of ventricular assist devices for the treatment of advanced heart failure in Japan was approved in 2000 with reimbursement of $31,600 (3,160,000¥) for external pulsatile device. As of November 2012, the HeartMate II implantable continuous-flow device was approved for use in Japan with reimbursement announced April 2013 at a rate of $180,000 (18,100,000¥) [16] (Table 4.1; Japanese VAD Reimbursement Rates).
Table 4.1
Japanese reimbursement for VADs
Functional category: VAD set | Functional code | Fee $US (¥yen) | ||||
|---|---|---|---|---|---|---|
(1) | Extracorporeal | B002 | 129 | 01 | ~$38,900 (¥3,130,000) | |
(2) | Implantable (pulsatile) | B002 | 129 | 02 | ~$172,600 (¥13,900,000) | |
(3) | Implantable (non-pulsatile) 1. Magnetic levitation type 2. Water circulation type | B002 B002 | 129 129 | 03 03 | 01 02 | ~$224,800 (¥18,100,000) ~$224,800 (¥18,100,000) |
(4) | Water circulation circuit set | B002 | 129 | 04 | ~$13,000 (¥1,050,000) | |
At this time, there is no Japanese LVAD cost data, but if we see a similar trend as reported in the USA, with experience, careful patient selection and improved survival, reduction of length of stay days, and readmissions, cost will go down (Table 4.2).
Table 4.2
Impact of VAD adverse event on cost
Adverse event | Incremental costs |
|---|---|
Impact of adverse event on cost | |
No complications | $147,722 |
Late bleeding (after 24 h) | $52,537 |
Respiratory failure | $38,076 |
Perioperative bleeding | $21,502 |
Infection (other than sepsis and pump housing infection) | $37,721 |
4.3 Cost-Effectiveness
It is important to understand the concept of a cost-effective analysis (CEA). The American College of Physicians explains how the CEA results might be considered as the “price” of the additional outcome purchased by switching from current practice to the new strategy. If the price is low enough, the new strategy is considered “cost-effective.” If a strategy is dubbed “cost-effective” and the term is used as its creators intended, it means that the new strategy is a good value. Being cost-effective does not mean the strategy saves money, and just because a strategy saves money doesn’t mean that it is cost-effective. The very notion of cost-effective requires a value judgment—what you think is a good price for an additional outcome, someone else may not. What is life worth? [17].
The clinical assessment of the left ventricular device technology is evolving. It has been clearly established as clinically beneficial with a favorable technology assessment from Blue Cross Blue Shield (BCBS) Technology Assessment Committee followed by positive National Coverage Determination by CMS as the treatment for end-stage heart failure patients ineligible for heart transplant also known as destination therapy (DT) [18, 19].
The overall clinical acceptance of this therapy led to an immediate review by BCBS on whether the use of the therapy was a cost-efficient treatment. These initial studies suggested LVADs were not cost-effective, with LVAD incremental cost-effectiveness ratio (ICER) “price” as high as $802,700 (BCBS TEC), which is beyond generally accepted threshold of $50,000–100,000 per quality-adjusted life year (QALY) “value” gained [20].
More recently, an assessment of patients undergoing both acute and chronic VAD support with multiple types of first-generation mechanical assist devices suggested it was a high-cost therapy that did not meet Institute of Medicine goals for cost or outcomes when costs were analyzed out to 1 year post implant. The problem with retrospective data compiled over a number of years in a field that is evolving so rapidly is that it does not reflect current practice or outcomes [21, 22].
Using a more recent dataset from the HeartMate II Destination Therapy trial that included initial hospital stay, outpatient supplies, re-hospitalizations, and Medicare payments for professional services, the cost of LVAD therapy was compared with the cost of medical therapy to treat advanced heart failure patients. Continuous-flow LVAD patients had higher quality-adjusted life years (1.87 versus 0.37), and life years (2.42 versus 0.64), as well as higher 5-year costs ($360,407 versus $62,856) for medical management. The incremental cost-effectiveness ratio of the continuous-flow LVAD was $198,184 per QALY and $167,208 per life year, which was equivalent to a relative 75 % reduction in incremental cost-effectiveness ratio from $802, 700 per QALY in 2004. [23] This recent data demonstrate LVAD therapy has not yet achieved cost-effectiveness (strictly as per definition) in the USA (<$100,000/incremental cost-effectiveness ratio), but the improvements gained in the short term are encouraging.
The primary determinants of cost-effectiveness using current methodology are total cost, survival, and quality of life. If quality of life remains stable, combinations of cost stabilize and 2-year survival improves from 58 to 70%, and then using the same cost-effective model, the HeartMate II would meet the $100,000 ICER [24] when used as destination therapy.
However, one important deficiency in using only cost in the assessment of the effectiveness of lifesaving therapies is it omits the very important parameters of change in functional capacity and life management. The improvement in functional capacity with medical therapy for advanced heart failure is modest at best. While cardiac resynchronization therapy has been adopted as an accepted treatment of patients with advanced heart failure who meet eligibility criteria, the improvement reported at 6 months on the 6-minute walk test (6-MWT) in three recent studies was only 42 m [25, 26]. In contrast, the average increase in 6-MWT following use of a new continuous-flow design LVAD was nearly 300 m. In addition, the improvement in patient-assessed quality of life in the LVAD trial at 3 and 6 months was increased nearly 90 % from baseline and higher than has been reported for any medical therapy. Cost-effective models do not completely capture such pertinent elements as the actual quality impact to patients’ lives [27].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


