Fig. 12.1
Antoine Laurent Lavoisier (1747–1794) with his wife Marie-Anne (1759–1836). On the far left is the drawing board used by Marie-Anne. On the far right is a glass jar containing water and resting on a porcelain dish. This jar was presumably used for collecting gas. Next to it is a narrow tube partly filled with mercury. This is a eudiometer for measuring the oxygen concentration of gases. To the left of that is a mercury receiver for collecting gas. On the floor there is a large glass vessel that was used for experiments to make water from hydrogen and oxygen. Next to it is a hydrometer for measuring the specific gravity of fluids. This magnificent portrait by Jacques-Louis David (1780) is in the Metropolitan Museum of Art, New York. Reproduced by permission
Because of his eminence, there is a vast literature on Lavoisier’s scientific accomplishments. For readers of this article, Holmes [8] is recommended for the physiology, and McKie [17] for the chemistry. Beretta [1, 2] and Prinz [22, 23] are informative about Marie-Anne’s drawings. Guerlac [7] is a comprehensive database, and Grimaux [6] is a standard biography in French. The web site Panopticon Lavoisier [20] is exhaustive. The present article concentrates on Lavoisier’s main contributions to respiratory physiology, particularly the first measurements of human oxygen consumption, with a major emphasis on the important role of Marie-Anne Lavoisier which has often been overlooked.
12.2 Antoine Lavoisier’s Contributions to Respiratory Physiology
1.
Identification of the three respiratory gases
Lavoisier was the first person to clearly state the role of oxygen, carbon dioxide and nitrogen in respiration. Here he built on the previous work of other investigators particularly Priestley. This English non-Conformist minister carried out an experiment in August 1774 when, on heating some red mercuric oxide, he found that a remarkable gas was produced. He stated “but what surprized me more than I can yet well express, was that a candle burned in this air with a remarkably vigorous flame …and a piece of red-hot wood sparkled in it” [21]. Furthermore he showed that a mouse was able to survive longer in this gas than in ordinary air, and he famously surmised that it might be useful for people with disease. Thus there was no doubt that Priestley had produced oxygen. However unfortunately he did not understand its nature. Priestley was a follower of the phlogiston theory that had been promoted by Georg Ernst Stahl (1659–1734) and that stated that all combustible materials are composed of ash (calx) and phlogiston (Greek for inflammable), and that during burning, phlogiston escaped leaving the dephlogisticated ash behind. This is in fact the reverse of what happens during combustion when oxygen combines with a combustible material, but the theory was enormously influential in the mid-eighteenth century. Priestley is often credited with “discovering” oxygen just as Columbus “discovered” America but neither Priestley nor Columbus correctly identified what they found.
Carl Wilhelm Scheele (1742–1786) in Sweden had independently produced oxygen even before Priestley although the publication of his findings was delayed [24]. Scheele called the gas “fire-air” but again he was influenced by the phlogiston theory and did not recognize its true nature.
Priestley visited Paris in October 1774 with his patron Lord Shelburne and they had dinner with Lavoisier and some other chemists. At that time Priestley described the experiments that he had carried out with mercuric oxide and naturally these evoked great interest. As a result, Lavoisier repeated the experiments and he was eventually able to understand the chemical processes. For example in 1775 in what became known as the Easter Memoir, Lavoisier stated that “the substance which combines with metals during calcination, thereby increasing their weight, is nothing else than the pure portion of the air which surrounds us and which we breathe”. This was the coup de grâce to the phlogiston theory. As we shall see later, Marie-Anne Lavoisier played an important part in this advance because it was she who translated the English texts of Priestley and another proponent of phlogiston, Richard Kirwan (1733–1812), so that Antoine could read them.
In 1777 Lavoisier communicated a memoir to the French Académie des Sciences titled Expériences sur la respiration des animaux, et sur les changements qui arrivent à l’air en passant par leur poumon [11]. This included the statement “Eminently respirable air [he later called it oxygine] that enters the lung, leaves it in the form of chalky aeriform acids [carbon dioxide] … in almost equal volume…. Respiration acts only on the portion of pure air that is eminently respirable … the excess, that is its mephitic portion [nitrogen], is a purely passive medium which enters and leaves the lung … without change or alteration. The respirable portion of air has the property to combine with blood and its combination results in its red color”. This arresting statement was the foundation of all subsequent work on the respiratory gases. Incidentally although a summary like this might suggest that Lavoisier’s development of ideas proceeded in a logical sequence, this was far from the case. He was frequently led off on some line of reasoning that turned out to be erroneous, and the whole process was extremely tortured.
2.
The recognition of respiration as combustion
This advance was also based on the work of earlier scientists, particularly Robert Boyle (1627–1691) and John Mayow (1641–1679). Lavoisier’s work was greatly assisted by his development of an ice calorimeter shown in Fig. 12.2. This beautiful engraving was done by Marie-Anne Lavoisier and is another example of her important colloboration. The calorimeter consisted of three compartments, one inside the other. In the center there was a space for a burning flame or a small animal such as a guinea pig. Surrounding that was a compartment containing ice, and the amount of water that was produced when this melted was a measure of the heat that was evolved. Finally there was an outer compartment containing ice that acted as an insulating jacket.
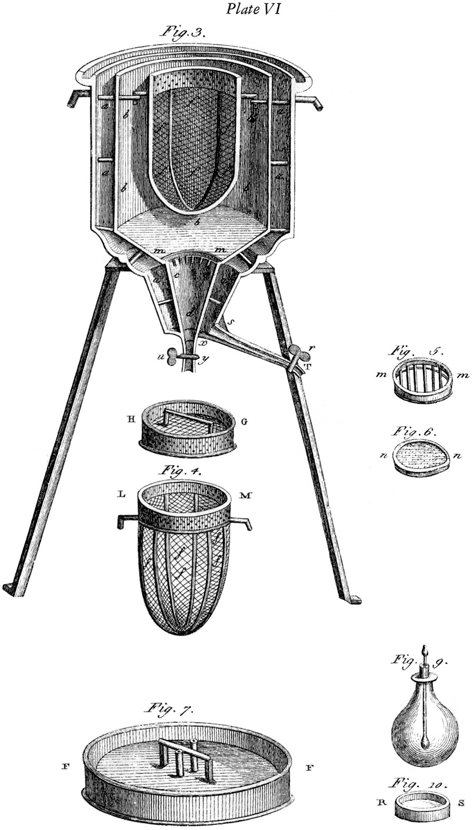
Fig. 12.2
Marie-Anne Lavoisier’s engraving of an ice calorimeter used by Lavoisier to demonstrate the similarity of respiration and combustion. See text for details. From [12]
Lavoisier was able to show that the heat evolved per unit of carbon dioxide produced was in the same ratio for both a flame and animals. He summarized his findings in the following striking sentence “La respiration n’est qu’une combustion lente de carbone et d’hydrogène, qui est semblable en tout à celle qui s’opère dans une lampe ou dans une bougie allumée, et que, sous ce point de vue, les animaux qui respirent sont de véritables corps combustibles qui brûlent et se consument”. [Respiration is nothing but a slow combustion of carbon and hydrogen, similar in all respects to that of a lamp or a lighted candle, and from this point of view, animals which breathe are really combustible substances burning and consuming themselves].
These experiments on combustion were carried out with a junior colleague Pierre-Simon de Laplace (1749–1827) and the two investigators made one of the few errors in Lavoisier’s work when they proposed that the combustion actually took place in the lungs. They stated “This combustion is produced within the lungs without evolving perceptible light … the heat developed in this combustion is communicated to the blood which traverses the lungs and is dispersed in the whole animal system” [14]. It is interesting that the site of energy metabolism proved to be very elusive for nearly 100 years after Lavoisier and there were often complicated discussions in textbooks under the heading “animal heat”.
3.
Measurements of human oxygen consumption under various conditions
Lavoisier and his collaborator, Armand Séguin (1767–1835) have the distinction of making the first human measurements of oxygen consumption. These were made during rest and exercise, and with the subject being exposed to different temperatures. These experiments are discussed in detail below together with the drawings by Marie-Anne Lavoisier.
12.3 Contributions of Marie-anne Lavoisier
Some historians of science have argued that Lavoisier’s work ranks him along with the other immortals such as Galileo, Newton, Darwin and Einstein. It is interesting to consider his wife’s contributions to his work in this context. Marie-Anne is perhaps unique in the sense that her collaborations with her famous husband were so important to his success. Certainly none of the other great scientists listed above had the same support from their spouses. Possible other contenders for this honor among respiratory physiologists in the twentieth century were August (1874–1949) and Marie Krogh (1874–1943).
Marie-Anne Lavoisier’s contributions fall into three main areas: translations, graphics, and editorship.
12.3.1 Marie-Anne Lavoisier’s Contributions Through Translations
As we shall see later, Marie-Anne found herself exposed to Lavoisier’s scientific career at a very early age and with little preparation. The couple were married on December 16, 1771, when Marie-Anne was only 13, about to turn 14. This was shortly after she had emerged from the convent where she received her education. However she was clearly highly intelligent and ambitious. Shortly after the marriage she became interested in the work of her husband and it was not long before she was being tutored in chemistry by one of Lavoisier’s colleagues, Jean-Baptiste Bucquet (1746–1780). He was an eminent chemist and physician, a member of the Académie des Sciences, and the author of several books. He had his own private laboratory from which he taught courses in chemistry. It was not surprising that Lavoisier had distinguished colleagues like this. Lavoisier himself had been elected to the Académie at the early age of 25 and through this had extensive contacts with many of the best scientists in France. Another of these was Jean-Hyacinthe de Magellan (1723–1790) who incidentally was a linear descendant of the great Portuguese navigator who discovered the passage to the Pacific Ocean that bears his name. In spite of his foreign-sounding name, Magellan resided in England and was a Fellow of the Royal Society. In a letter to Lavoisier in 1775 he referred to Marie-Anne as a “philosophical wife”.
At this time much of the most influential work on the chemistry of the respiratory gases was being done in England. The critical experiments of Priestley who first isolated oxygen have already been referred to. Another active scientist was Joseph Black (1728–1799) who worked in Scotland and clearly described carbon dioxide [3]. A further scientist was Henry Cavendish (1731–1810) who worked on the production of water from hydrogen and oxygen. It was essential for Lavoisier to have access to the results of these influential investigators and Marie-Anne assumed the responsibility. She set herself to learn English which Lavoisier himself never mastered, and her translations were of great importance in enabling Lavoisier to keep up with what was being done in England. Other writings by Marie-Anne in support of her husband’s work are also documented [9].
One important book translated by Marie-Anne was by Richard Kirwan titled Essay on Phlogiston and the Constitution of Acids and published in 1787 [10]. Kirwan was an influential scientist who was a Fellow of the Royal Society from which he received the prestigious Copley Medal. His book was one of the last and most detailed in the support of the theory of phlogiston. After Lavoisier had read the book he continued to debate the issue of phlogiston with Kirwan and associates for some time, but ultimately Kirwan conceded and acknowledged himself to be converted in 1791.
It was particularly important for Lavoisier to be aware of Priestley’s work because this enterprising scientist covered a lot of ground. As an example of his imaginative flair and the elegance of his writing, here is a brief excerpt. “My reader will not wonder, that, after having ascertained the superior goodness of the dephlogisticated air by mice living in it, and the other tests above mentioned, I should have the curiosity to taste it myself. I have gratified that curiosity, by breathing it, drawing it through a glass-syphon, and, by this means, I reduced a large jar to fit to the standard of common air. The feeling of it to my lungs was not sensibly different from that of common air; but I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell but that, in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it”. Priestley was clearly a man to be reckoned with.
12.3.2 Illustrations Including Engravings
One of Lavoisier’s major contributions to chemistry was to develop accurate quantitative procedures. For example he described the law of conservation of mass in chemical reactions by first weighing the reactants of a chemical reaction and subsequently weighing the products. To do this he needed to construct highly accurate balances. He also made careful measurements of the volumes of expired gas using pneumatic troughs or what we now call spirometers. These advances required the development of much new apparatus and Marie-Anne Lavoisier was responsible for making accurate illustrations of the new equipment. In Lavoisier’s major work Traité élémentaire de chimie of 1789, there are 13 exquisitely engraved plates by Marie-Anne. One example that was described earlier is shown in Fig. 12.2, and equipment for collecting gas is shown in Fig. 12.3. Note the extreme attention to detail including accurate dimensions that would allow other investigators to replicate the instruments. The engravings in Traité élémentaire de chimie are all grouped together at the end of the book and they provide essential information to complement the science described by Lavoisier. Some of Lavoisier’s experimental apparatus can be seen today in the Musée des Arts et Métiers in Paris.
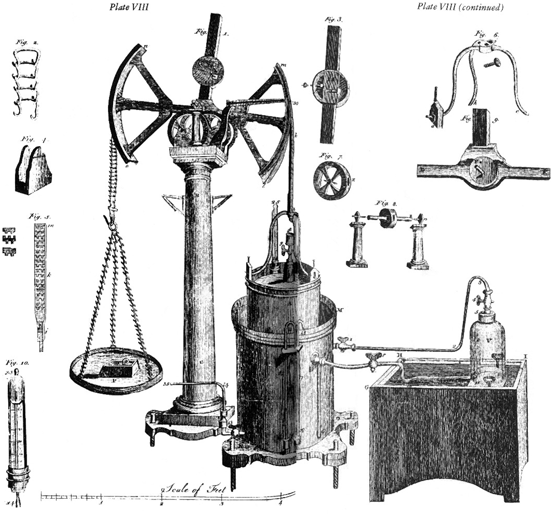
Fig. 12.3
Marie-Anne Lavoisier’s engraving of a device for collecting gas and measuring its volume. From [12]
Marie-Anne’s expertise in engraving is believed to have come from lessons with the famous artist Jacques-Louis David (1748–1825). He was one of the most influential painters in the neoclassical style in France in the late eighteenth century. Figure 12.1 shows one of his best known portraits and there is no equal in the portrayal of a famous scientist with his wife-collaborator. David painted many other famous historical events and his paintings are exhibited in major art galleries around the world.
As discussed below, Marie-Anne also worked on a major book of eight volumes entitled Mémoires de physique et chimie which was started by Lavoisier but interrupted by his execution. Surviving documents show that this also was planned to include a number of her engravings but the project was never completed [4].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


